TLDR: The action of insulin is the inhibition of lipolysis.
This is our next paper:
Sniffing neuropeptides: a transnasal approach to the human brain
This is from 2002. Intranasal insulin does interesting things. I just wanted to run through the initial results following a single dose of 40iu of intranasal insulin and a subsequent (successful!) weight loss study based on this dose. These are the levels of insulin in cerebrospinal fluid (CSF) of volunteers as measured by sampling through a spinal catheter placed at the L4-L5 level (ouch!) and also in plasma after that single 40iu intranasal dose. It strikes me that the the insulin concentration in the rostral CNS might be a bit higher than down at L4 but I guess no one would volunteer for cisternal punctures/catheter placement to check this:
CSF insulin hits 25pmol/l. There is no penetration in to the systemic circulation. My feeling is that a rise from a basal CSF insulin of 7.0pmol/l to a peak of 25pmol/l, ie just over a tripling of fasting levels, probably reflects what might well be a physiological post-prandial concentration change within the brain. Roughly.
Note that intranasal insulin enters the brain at a clearly detectable concentration within ten minutes.
Then we have this study, also based around that 40iu intra-nasal dose:
Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man
A set of subjects, all male (perhaps the word "man" in the title should read "men". I'll give you one guess as to whether this trick for weight loss works in women. Or in insulin resistant "man/men" either), took 40iu of intranasal insulin, four times daily, for eight weeks. This will have been on top of their normal pancreatic responses to food over this period. Or they took placebo. This is what happened:
What was the effect of the first dose of intra-nasal insulin? Or, rather, from the first 28 doses? Not a lot, judging by the weight change in the first week. Or the second week. Or the third week. That's 84 doses of intranasal insulin over three weeks. Recall 40iu of intranasal insulin triples CSF insulin concentration within ten minutes. No weight loss over three weeks. Does anyone else see a trend for weight gain in the treatment arm (filled circles) over the first three weeks or is that just my confirmation bias over riding my visual acuity?
Recall that intranasal insulin enters the brain within ten minutes...
Anyway. For three weeks, that's 84 doses of 40iu intranasal insulin, there is zero weight loss. Nil. Zilch. Nadder. If anything there is a minuscule trend upwards. And I mean minuscule.
Then by week four something marvellous happens and consistent weight loss ensues.
So the question is: How many 40iu intranasal doses of insulin does it take to induced VMH insulin resistance in young healthy men? Sadly the answer is not 42, this is merely the answer to Life, the Universe and Everything. It's actually twice that, 84.
It seems that it takes more like 84 CNS over-exposures to insulin to obtund its lipolysis inhibiting effect normally induced via the VMH. At this point nutrients are less effectively sequestered in to adipocytes and so become more available for metabolism, obtunding hunger and allowing weight loss.
If anyone has a better explanation for the shape of the weight loss graph I'd be interested to hear it. The authors don't.
Peter
Thursday, August 23, 2018
Tuesday, August 21, 2018
Another brief housekeeping post
OK. I published the "awaiting moderation" comments. Lots of them are insightful and need a response. Time for this is not looking good at the moment! The main upset is that I mis-clicked delete instead of publish on a comment by Nicolás Flamel which I can't find any way of recovering. Sorry for that. Please feel free to re comment if you wish.
Peter
Peter
Insulin makes you hungry (5) except when you resist
TLDR: The function of insulin is the inhibition of lipolysis. Does resisting insulin facilitate lipolysis?
This the next paper:
Improving Influence of Insulin on Cognitive Functions in Humans
Aside: Oooooh, look. No control group! How can I already tell they are going to find that insulin suppresses appetite? End aside, sniggering excepted.
The only parts I am interested in are the clamps and the appetite scores (no food intakes in this one).
So we have a low insulin clamp and a high insulin clamp looking a lot like this:
These are the hunger ratings during the clamps:
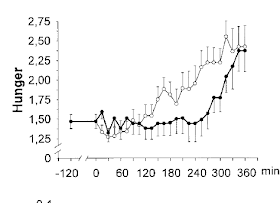
I think we can say that hunger kicked in at around 150 minutes under the low dose insulin clamp and at around 300 minutes in the high dose insulin clamp. When would hunger kick in w/o insulin? We'll never know because...
So anyway, there we have it. Insulin, entering the brain by the physiological route, suppresses appetite in real live humans with a dose response. Time to shut up shop and go back to kayaking in my free time.
But just one moment. I think it might be worth looking at this study in the units of insulin concentration that we are most used to working with. I used this website to do the conversions.
We have here a fasting insulin somewhere around 40-50pmol/l. In old money that is 6.0microU/ml, quite reasonable.
The low clamp is around 900pmol/l, just over 130microU/ml. This is pretty well "normal" for physiological post prandial insulin after eating a meal of junk.
The "high" insulin clamp is around 30,000pmol/l (it's a log scale) by the six hour mark. I'll try to be careful with decimal points here, but I think this equates to just over 4000microU/ml. I'm not sure that even an insulinoma patient would run an insulin of 4000microU/ml. If anyone thinks this sort of insulin level has anything to do with appetite control in the physiology of humans, then they may be mistaken. But this level of insulin exposure does delay the onset of hunger, by over two hours in this study...
Now, here is a sideways way of looking at CNS insulin.
If the physiological role of insulin in the VMH is to augment fat storage, what might be the effect of CNS insulin resistance? Go on. I dare you to say that the effect might be partial failure to suppress lipolysis, less suppression of FFAs and so reduced appetite augmentation. Resisting insulin allows you to resist its hunger generating effects. How's that for a bizarre idea? Feel free to point out faults in the logic.
I've long been interested in the concept that exposure to insulin itself induces insulin resistance. There are a whole slew of papers to suggest this, some better than others. Overall I find the concept quite convincing.
If we want to actually see insulin resistance kick in rapidly, and measure it, we have to go to cell culture. Here we can overdose by decent amounts, using nanomoles rather than picomoles, and watch the reduction in signalling triggered by these high insulin concentrations. Insulin receptor (IR) phosphorylation drops within 45 minutes from the initial peak at 10 minutes after exposure. It's in this paper if anyone is interested in the details
Insulin Resistance Induced by Hyperinsulinemia Coincides with a Persistent Alteration at the Insulin Receptor Tyrosine Kinase Domain
but this is a typical graph:
If we convert the nanomolar concnetration from cell culture to the picomoles of in-vivo clamps then the two higher concentrations used in this graph would be 17,000pmol and 170,000pmol, similar to those from the high dose clamp used in the human study. Especially when you consider that 17,000pmol and 170,000pmol have indistinguishable effects on IR phosphorylation (and acute generation of resistance to that phosphorylation effect) in cell culture.
So it seems quite feasible to me that a physiological clamp at 900pmol/l would produce normal "insulin hunger" due to lipolysis suppression and that a clamp at 30,000pmol/l would induce significant insulin resistance. That limitation on insulin signalling would obtund the normal inhibition of lipolysis (but not eliminate it entirely) and so defer the normal onset of hunger routinely induced by the physiological action of insulin. So very large doses of insulin (170,000pmol in cell culture imitating 30,000pmol/l as a clamp) should postpone the onset of hunger compared to mildly supra-physiological doses (5000pmol in culture imitating 900pmol/l as a clamp) because the higher doses should cause less inhibition of lipolysis, with the proviso that you choose your target concentration very carefully. Obviously no one does a study without a pilot trial to make sure they will get the results they want. A saline infusion produces no significant increase in hunger over 150 minutes, though the trend in hunger is upwards because supper was a long time ago by the end of that particular study!
I'll take a pause here because I think this current concept is quite controversial enough to be stated as simply as possible. Obviously there are more studies which we can look at to explore the usefulness of this idea.
Peter
This the next paper:
Improving Influence of Insulin on Cognitive Functions in Humans
Aside: Oooooh, look. No control group! How can I already tell they are going to find that insulin suppresses appetite? End aside, sniggering excepted.
The only parts I am interested in are the clamps and the appetite scores (no food intakes in this one).
So we have a low insulin clamp and a high insulin clamp looking a lot like this:
These are the hunger ratings during the clamps:
I think we can say that hunger kicked in at around 150 minutes under the low dose insulin clamp and at around 300 minutes in the high dose insulin clamp. When would hunger kick in w/o insulin? We'll never know because...
So anyway, there we have it. Insulin, entering the brain by the physiological route, suppresses appetite in real live humans with a dose response. Time to shut up shop and go back to kayaking in my free time.
But just one moment. I think it might be worth looking at this study in the units of insulin concentration that we are most used to working with. I used this website to do the conversions.
We have here a fasting insulin somewhere around 40-50pmol/l. In old money that is 6.0microU/ml, quite reasonable.
The low clamp is around 900pmol/l, just over 130microU/ml. This is pretty well "normal" for physiological post prandial insulin after eating a meal of junk.
The "high" insulin clamp is around 30,000pmol/l (it's a log scale) by the six hour mark. I'll try to be careful with decimal points here, but I think this equates to just over 4000microU/ml. I'm not sure that even an insulinoma patient would run an insulin of 4000microU/ml. If anyone thinks this sort of insulin level has anything to do with appetite control in the physiology of humans, then they may be mistaken. But this level of insulin exposure does delay the onset of hunger, by over two hours in this study...
Now, here is a sideways way of looking at CNS insulin.
If the physiological role of insulin in the VMH is to augment fat storage, what might be the effect of CNS insulin resistance? Go on. I dare you to say that the effect might be partial failure to suppress lipolysis, less suppression of FFAs and so reduced appetite augmentation. Resisting insulin allows you to resist its hunger generating effects. How's that for a bizarre idea? Feel free to point out faults in the logic.
I've long been interested in the concept that exposure to insulin itself induces insulin resistance. There are a whole slew of papers to suggest this, some better than others. Overall I find the concept quite convincing.
If we want to actually see insulin resistance kick in rapidly, and measure it, we have to go to cell culture. Here we can overdose by decent amounts, using nanomoles rather than picomoles, and watch the reduction in signalling triggered by these high insulin concentrations. Insulin receptor (IR) phosphorylation drops within 45 minutes from the initial peak at 10 minutes after exposure. It's in this paper if anyone is interested in the details
Insulin Resistance Induced by Hyperinsulinemia Coincides with a Persistent Alteration at the Insulin Receptor Tyrosine Kinase Domain
but this is a typical graph:
If we convert the nanomolar concnetration from cell culture to the picomoles of in-vivo clamps then the two higher concentrations used in this graph would be 17,000pmol and 170,000pmol, similar to those from the high dose clamp used in the human study. Especially when you consider that 17,000pmol and 170,000pmol have indistinguishable effects on IR phosphorylation (and acute generation of resistance to that phosphorylation effect) in cell culture.
So it seems quite feasible to me that a physiological clamp at 900pmol/l would produce normal "insulin hunger" due to lipolysis suppression and that a clamp at 30,000pmol/l would induce significant insulin resistance. That limitation on insulin signalling would obtund the normal inhibition of lipolysis (but not eliminate it entirely) and so defer the normal onset of hunger routinely induced by the physiological action of insulin. So very large doses of insulin (170,000pmol in cell culture imitating 30,000pmol/l as a clamp) should postpone the onset of hunger compared to mildly supra-physiological doses (5000pmol in culture imitating 900pmol/l as a clamp) because the higher doses should cause less inhibition of lipolysis, with the proviso that you choose your target concentration very carefully. Obviously no one does a study without a pilot trial to make sure they will get the results they want. A saline infusion produces no significant increase in hunger over 150 minutes, though the trend in hunger is upwards because supper was a long time ago by the end of that particular study!
I'll take a pause here because I think this current concept is quite controversial enough to be stated as simply as possible. Obviously there are more studies which we can look at to explore the usefulness of this idea.
Peter
Friday, August 03, 2018
Holiday reading
I'm off on vacation for a while so the next posts are likely to be delayed. If anyone would like a light summary of the ideas I'm thinking about, this is a nice review:
Yin and Yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism
For a level deeper of understanding you just need to add in that saturated fats have an FADH2:NADH ratio around 0.49 which is the physiological signal for insulin resistance and MUFA generate one of around 0.47, giving the signal for insulin sensitivity. This is physiology.
Adding in PUFA as a bulk calorie source, with an insulin hyper-sensitivity generating FADH2:NADH ratio of well below 0.47, leads to expanded adipocytes. This is pathology.
Because a core function of insulin is the inhibition of lipolysis.
Peter
Yin and Yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism
For a level deeper of understanding you just need to add in that saturated fats have an FADH2:NADH ratio around 0.49 which is the physiological signal for insulin resistance and MUFA generate one of around 0.47, giving the signal for insulin sensitivity. This is physiology.
Adding in PUFA as a bulk calorie source, with an insulin hyper-sensitivity generating FADH2:NADH ratio of well below 0.47, leads to expanded adipocytes. This is pathology.
Because a core function of insulin is the inhibition of lipolysis.
Peter
Wednesday, August 01, 2018
Insulin makes you hungry (4) unless you keep it out of your brain
TLDR: The function of insulin is the inhibition of lipolysis. Especially via the brain. Where insulin detemir doesn't go.
People will be aware that insulin detemir is really strange stuff. There are perfectly respectable papers showing that it cannot enter the brain (and blocks the entry of normal insulin in to the brain too) or that it is fantastic at entering the brain, much better than more normal insulins.
There are probably more studies in the latter camp but my biases push me towards the former camp. The nature of the researchers also tends to push me towards the former camp. I posted on insulin detemir here and here to explain my point of view.
Now there is this paper:
Euglycemic Infusion of Insulin Detemir Compared With Human Insulin Appears to Increase Direct Current Brain Potential Response and Reduces Food Intake While Inducing Similar Systemic Effects
OK. After an overnight fast, a 90 minute euglycaemic hyperinsulinaemic clamp and a 20 minute wait, subjects consistently eat less food (303kcal, 17% reduction) after an insulin detemir clamp (1475kcal) than they do after a neutral insulin clamp (1782kcal). Just eyeballing the insulin doses used we can assume that the plasma insulin levels were a reasonable approximation for humans in the normal post prandial period, ie physiological fed-state rather than pharmacological.
The research group is completely wedded to the idea that central insulin is an appetite suppressant and that weight gain from any insulin therapy is only a reaction to recurrent hypoglycamia. As there is no hypoglycaemia during the clamps their presumption is that this neutral insulin infusion results in a reduced food intake. As insulin detemir gives less food intake after a normoglycaemic clamp than neutral insulin does, then their conclusion is that insulin detemir is having a more potent central appetite suppressing effect than the neutral insulin.
They are so confident about this that the inclusion of a control situation, where saline was infused without any insulin and appetite was checked after this, was considered un-necessary. This really is the level of research in the "satiety" insulin camp.
Fortunately for us we do still have results from 1985 where food intake after a physiologoical post-prandial level clamp at 150microU/ml for 150 minutes using neutral insulin was compared to saline control and these give us this table cited in a previous post ("liquid drink" is a calorie containing soup-like food):
which allows us to calculate that saline reduces food intake by 40% compared to neutral insulin. Or to rephrase that more plausibly: a clamp using neutral insulin increases food intake by 60%. You can see why a control group was omitted by the "satiety from insulin" paper. I rather like insulin determir compared to any other insulin but you can see it has its work cut out to beat saline as a satiety hormone!
Simplest explanation: Insulin in the brain decreases peripheral lipolysis. This makes you hungry after an hyperinsulinaemic clamp. Insulin detemir doesn't enter the brain so has no CNS augmentation of its peripheral suppressive effect on lipolysis at a given level of glucose control, so it generates less hunger.
I'm a simple sort of a person. That's how I see it. Could be wrong of course.
Peter
People will be aware that insulin detemir is really strange stuff. There are perfectly respectable papers showing that it cannot enter the brain (and blocks the entry of normal insulin in to the brain too) or that it is fantastic at entering the brain, much better than more normal insulins.
There are probably more studies in the latter camp but my biases push me towards the former camp. The nature of the researchers also tends to push me towards the former camp. I posted on insulin detemir here and here to explain my point of view.
Now there is this paper:
Euglycemic Infusion of Insulin Detemir Compared With Human Insulin Appears to Increase Direct Current Brain Potential Response and Reduces Food Intake While Inducing Similar Systemic Effects
OK. After an overnight fast, a 90 minute euglycaemic hyperinsulinaemic clamp and a 20 minute wait, subjects consistently eat less food (303kcal, 17% reduction) after an insulin detemir clamp (1475kcal) than they do after a neutral insulin clamp (1782kcal). Just eyeballing the insulin doses used we can assume that the plasma insulin levels were a reasonable approximation for humans in the normal post prandial period, ie physiological fed-state rather than pharmacological.
The research group is completely wedded to the idea that central insulin is an appetite suppressant and that weight gain from any insulin therapy is only a reaction to recurrent hypoglycamia. As there is no hypoglycaemia during the clamps their presumption is that this neutral insulin infusion results in a reduced food intake. As insulin detemir gives less food intake after a normoglycaemic clamp than neutral insulin does, then their conclusion is that insulin detemir is having a more potent central appetite suppressing effect than the neutral insulin.
They are so confident about this that the inclusion of a control situation, where saline was infused without any insulin and appetite was checked after this, was considered un-necessary. This really is the level of research in the "satiety" insulin camp.
Fortunately for us we do still have results from 1985 where food intake after a physiologoical post-prandial level clamp at 150microU/ml for 150 minutes using neutral insulin was compared to saline control and these give us this table cited in a previous post ("liquid drink" is a calorie containing soup-like food):
which allows us to calculate that saline reduces food intake by 40% compared to neutral insulin. Or to rephrase that more plausibly: a clamp using neutral insulin increases food intake by 60%. You can see why a control group was omitted by the "satiety from insulin" paper. I rather like insulin determir compared to any other insulin but you can see it has its work cut out to beat saline as a satiety hormone!
Simplest explanation: Insulin in the brain decreases peripheral lipolysis. This makes you hungry after an hyperinsulinaemic clamp. Insulin detemir doesn't enter the brain so has no CNS augmentation of its peripheral suppressive effect on lipolysis at a given level of glucose control, so it generates less hunger.
I'm a simple sort of a person. That's how I see it. Could be wrong of course.
Peter