Tucker and Mike both gave me the heads-up on this paper recently.
Linoleic acid causes greater weight gain than saturated fat without hypothalamic inflammation in the male mouse
It's nice and simple: feeding 22.5% of calories as linoleic acid (LA) to a mouse makes it obese and insulin resistant. Feeding 15% of calories as LA makes it identically obese but without the insulin resistance. The argument can be made, very convincingly, that LA is converts to 4-hydroxynonenyl (4-HNE) in proportion to the LA content of the diet. 4-HNE is a powerful driver of insulin resistance so the hypoglycaemic response to exogenous insulin is markedly blunted in the 22.5% LA group of mice. As in the top line here:
Here are the weight gains, which need a little consideration:
With the eye of faith you can see that the top line (22.5% LA) starts off gaining weight faster than the next line down (15% LA) but converges from around 7-8 weeks onward. My guess is that this is when insulin resistance from 4-HNE started to over-ride the insulin sensitising effect of LA which caused the weight gain in the first place.
Now the third line down is the interesting one. This diet only contained 1% linoleic acid. OK, these mice are statistically significantly slimmer than the 15% and 22.5% LA fed mice (p less than 0.05) but they are hardly exactly svelte when compared to the crapinabag (CIAB)-fed control mice (black line down at the bottom). And the CIAB food contained 4.22% of calories derived from LA.
That needs some thinking about.
It makes me ask: Why do the vast majority of high fat fed mice/rats become obese? Apart from the fact that they have been selected for this response.
There's probably another post or two needed on that one.
Peter
As an aside I would just comment that while I agree with Tucker that 4-HNE and related products of free radical modified PUFA are the best explanation for this study, my feeling is that the 15% LA group with sustained insulin sensitivity allowing sustained weight gain probably explains the situation in the current human population rather better. As sustained adipocyte distension progresses then we eventually get FFAs released in the presence of glucose and insulin, a Bad Thing. The ROS from this combination will eventually generate 4-HNE too but rather further down the obesity road compared with the 22.5% LA situation.
Monday, December 31, 2018
Thursday, December 20, 2018
Urinary c-peptide
It's the Winter Solstice tomorrow, greetings to all! My favourite astronomical event of the year, even though we do the major feasting on Christmas day in our house. Anyway, here's a fairytale for the depths of Winter (in the northern hemisphere anyway). Happy Solstice!
*********
Here we go: Just occasionally you come across a statement like this:
"(B) Insulin secretion throughout the day was assessed by 24-hr urinary c-peptide excretion and was significantly reduced only following the RC diet".
Okay, what does it suggest to us when the reduced 24-hr urinary c-peptide group lost less fat than the higher c-peptide group? Less insulin but less fat loss??? Perhaps it suggests that the insulin hypothesis of obesity is incorrect?
C-peptide is part of pro-insulin. Each molecule of insulin produced provides one molecule of c-peptide within the pancreas. Assuming (not completely accurately) that c-peptide is not consumed within the body we can use its 24-hr average urinary excretion as a surrogate for overall insulin production. With an awful lot of caveats, this seems fair to me. I think statement B is correct.
So 24-hr urinary c-peptide gives us an idea of how many molecules of insulin are being manufactured per day by the islets within the pancreas. Insulin is broken down by insulin degrading enzyme as part of its signalling process, not exactly proportionally, but as a general principle this is correct. I went through it in some detail when thinking about the Potato Diet, a sub-category of carbosis. The more signalling, the more degradation.
On average around 50% of secreted insulin (in dogs on a mixed diet) is removed by the liver on first passage from the portal vein through to the hepatic vein (termed first past extraction, FPE). Humans are very similar. None of this hepatic first pass extracted insulin ever arrives in the general circulation. The rate of extraction varied from as low as just over 20% up to almost 80% in the dog study. If you have 100 molecules of c-peptide produced, somewhere between 20 and 80 of the associated molecules of insulin will never arrive in the systemic circulation.
Does anything specific alter the FPE? Well, yes, of course. Does anyone think it might be free fatty acid delivery to the liver? Much as this paper tells us
Free Fatty Acids Impair Hepatic Insulin Extraction in Vivo
So under a weight stable modest LC diet (or more accurately; under whole body adipose tissue mass stability) the reduction of insulin secretion from the pancreas under that modestly reduced carbohydrate intake will undoubtedly occur, but would be offset by reduced hepatic FPE and enough insulin will penetrate to adipocytes to keep them full. In the real world this is extremely difficult to make happen, people just want to eat less on a LC diet because as insulin falls more fat exits adipocytes and hunger diminishes. As in Aberdeen. But you can approximate it by artificially controlling (increasing) food intake, ie you pay people to eat more dietary fat than they would like to (which keeps insulin secretion unchanged but increases fatty acid delivery to the liver), hepatic FPE falls and more insulin reaches adipocytes to keep them full.
Under ketosis (let's say with carbs less than 20g/d) there is so little insulin secretion that having an FPE which is probably approaching zero doesn't matter much. Near basal physiological insulin is secreted, almost none is FPE-ed by the liver but there is still minimal exposure of adipocytes to insulin because almost none is being secreted in the first place. So appetite plummets as FFAs rise as they pour out from the adipocytes, despite a minimal hepatic FPE. This should make it even harder to overeat. However, if you do manage it, minimal hepatic FPE by the liver is one of your methods to maintain fat stores under ketosis!
Conversely you can achieve low FFA delivery to the liver by using acipimox. People do not necessarily gain weight with this lipolysis inhibiting drug because it decreases hepatic FFA delivery, so increases hepatic insulin signalling and so increases hepatic insulin metabolism. I assume this increased insulin metabolism will increase FPE and this will decrease insulin delivery to the systemic circulation. I also think this is also how carbosis works, hence the need for very low fat provision in any carbosis inducing diet.
Anyway, here's a thought experiment using made-up numbers. Any resemblance to real life is totally accidental. If a moderate carb diet (say 140g/d) allows a 22% fall in 24-hr urinary c-peptide, does this mean there is a 22% fall in 24h exposure of adipocytes to systemic insulin? Well no...
Say 100 molecules of insulin are secreted and FPE pre-study is 50% then 50 molecules of insulin survive passage through the liver to suppress systemic lipolysis. If only 78 molecules of insulin are secreted under mild carbohydrate restriction but FPE falls to 20% (exaggeration to make the point!) due to increased lipolysis delivering extra FFAs to the liver, then 62 molecules of insulin will make it through FPE and as far as the adipocytes. This insulin could conceivably allow less lipolysis when compared to a carbosis inducing diet, despite reducing insulin secretion.
On a diet in which fat is so restricted as to allow almost none to be spared for oxidation so that FFA delivery to the liver falls precipitously, we can suggest an 80% FPE might be the result. This would be the situation under carbosis, say with a human eating 7.7% fat as part of a severely calorie restricted diet. So of the 100 molecules of insulin still being produced under these circumstances (typified by no fall in 24-hr urinary c-peptide) with an 80% FPE only 20 of those molecules of insulin will eventually hit the adipocytes and so lipolysis would then be greater than under the modest LC diet.
Please bear in mind that these numbers are a reductio ad absurdum example, but they do make a point about what is possible. There are other effects which would kick in but that's not my point here.
My grossly biased opinion is that any study which shows an intervention with superiority in fat loss will be associated with either lower insulin exposure of adipocytes or with an induced failure of insulin to act on those adipocytes (ie by metformin, alcohol, fructose or of course palmitic acid, plus a few others) than is achieved by any comparison diet.
But then I would say that..........
Peter
*********
Here we go: Just occasionally you come across a statement like this:
"(B) Insulin secretion throughout the day was assessed by 24-hr urinary c-peptide excretion and was significantly reduced only following the RC diet".
Okay, what does it suggest to us when the reduced 24-hr urinary c-peptide group lost less fat than the higher c-peptide group? Less insulin but less fat loss??? Perhaps it suggests that the insulin hypothesis of obesity is incorrect?
C-peptide is part of pro-insulin. Each molecule of insulin produced provides one molecule of c-peptide within the pancreas. Assuming (not completely accurately) that c-peptide is not consumed within the body we can use its 24-hr average urinary excretion as a surrogate for overall insulin production. With an awful lot of caveats, this seems fair to me. I think statement B is correct.
So 24-hr urinary c-peptide gives us an idea of how many molecules of insulin are being manufactured per day by the islets within the pancreas. Insulin is broken down by insulin degrading enzyme as part of its signalling process, not exactly proportionally, but as a general principle this is correct. I went through it in some detail when thinking about the Potato Diet, a sub-category of carbosis. The more signalling, the more degradation.
On average around 50% of secreted insulin (in dogs on a mixed diet) is removed by the liver on first passage from the portal vein through to the hepatic vein (termed first past extraction, FPE). Humans are very similar. None of this hepatic first pass extracted insulin ever arrives in the general circulation. The rate of extraction varied from as low as just over 20% up to almost 80% in the dog study. If you have 100 molecules of c-peptide produced, somewhere between 20 and 80 of the associated molecules of insulin will never arrive in the systemic circulation.
Does anything specific alter the FPE? Well, yes, of course. Does anyone think it might be free fatty acid delivery to the liver? Much as this paper tells us
Free Fatty Acids Impair Hepatic Insulin Extraction in Vivo
So under a weight stable modest LC diet (or more accurately; under whole body adipose tissue mass stability) the reduction of insulin secretion from the pancreas under that modestly reduced carbohydrate intake will undoubtedly occur, but would be offset by reduced hepatic FPE and enough insulin will penetrate to adipocytes to keep them full. In the real world this is extremely difficult to make happen, people just want to eat less on a LC diet because as insulin falls more fat exits adipocytes and hunger diminishes. As in Aberdeen. But you can approximate it by artificially controlling (increasing) food intake, ie you pay people to eat more dietary fat than they would like to (which keeps insulin secretion unchanged but increases fatty acid delivery to the liver), hepatic FPE falls and more insulin reaches adipocytes to keep them full.
Under ketosis (let's say with carbs less than 20g/d) there is so little insulin secretion that having an FPE which is probably approaching zero doesn't matter much. Near basal physiological insulin is secreted, almost none is FPE-ed by the liver but there is still minimal exposure of adipocytes to insulin because almost none is being secreted in the first place. So appetite plummets as FFAs rise as they pour out from the adipocytes, despite a minimal hepatic FPE. This should make it even harder to overeat. However, if you do manage it, minimal hepatic FPE by the liver is one of your methods to maintain fat stores under ketosis!
Conversely you can achieve low FFA delivery to the liver by using acipimox. People do not necessarily gain weight with this lipolysis inhibiting drug because it decreases hepatic FFA delivery, so increases hepatic insulin signalling and so increases hepatic insulin metabolism. I assume this increased insulin metabolism will increase FPE and this will decrease insulin delivery to the systemic circulation. I also think this is also how carbosis works, hence the need for very low fat provision in any carbosis inducing diet.
Anyway, here's a thought experiment using made-up numbers. Any resemblance to real life is totally accidental. If a moderate carb diet (say 140g/d) allows a 22% fall in 24-hr urinary c-peptide, does this mean there is a 22% fall in 24h exposure of adipocytes to systemic insulin? Well no...
Say 100 molecules of insulin are secreted and FPE pre-study is 50% then 50 molecules of insulin survive passage through the liver to suppress systemic lipolysis. If only 78 molecules of insulin are secreted under mild carbohydrate restriction but FPE falls to 20% (exaggeration to make the point!) due to increased lipolysis delivering extra FFAs to the liver, then 62 molecules of insulin will make it through FPE and as far as the adipocytes. This insulin could conceivably allow less lipolysis when compared to a carbosis inducing diet, despite reducing insulin secretion.
On a diet in which fat is so restricted as to allow almost none to be spared for oxidation so that FFA delivery to the liver falls precipitously, we can suggest an 80% FPE might be the result. This would be the situation under carbosis, say with a human eating 7.7% fat as part of a severely calorie restricted diet. So of the 100 molecules of insulin still being produced under these circumstances (typified by no fall in 24-hr urinary c-peptide) with an 80% FPE only 20 of those molecules of insulin will eventually hit the adipocytes and so lipolysis would then be greater than under the modest LC diet.
Please bear in mind that these numbers are a reductio ad absurdum example, but they do make a point about what is possible. There are other effects which would kick in but that's not my point here.
My grossly biased opinion is that any study which shows an intervention with superiority in fat loss will be associated with either lower insulin exposure of adipocytes or with an induced failure of insulin to act on those adipocytes (ie by metformin, alcohol, fructose or of course palmitic acid, plus a few others) than is achieved by any comparison diet.
But then I would say that..........
Peter
Wednesday, December 12, 2018
A post not about Walter Kempner
I've had this paper on my hard drive for a while. It's been sitting somewhere near the front of the back of my mind but was doing nothing to really grab my interest.
Chronic high-sucrose diet increases fibroblast growth factor 21 production and energy expenditure in mice
I've got a draft of a post from mid summer this year which I wrote simply because I like the attitude of the authors. They say things like:
"Excess carbohydrate intake causes obesity in humans".
That's the first line of the abstract. You know, it's that "nailing your colours to the mast" sort of a statement. Even though I do think life is a little more complex than that.
Anyway, I like these folks who are looking at the slimming effect of sucrose in BL6 mice. That's correct, sucrose is a slimming drug/food in mice, under the correct circumstances. People too. The data in the 2017 paper is an extension of the work they did in 2012, written up in this paper:
Ingestion of a moderate high‐sucrose diet results in glucose intolerance with reduced liver glucokinase activity and impaired glucagon‐like peptide‐1 secretion
I don't intend to go through either paper in detail, it's just that the 2012 paper has some rather special macro ratios that caught my eye.
This is what they did to the mice in that original paper:
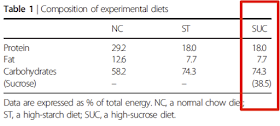
"After adaptation for 2 weeks, they [the mice] were divided into three groups and fed a normal chow diet (NC), a high‐starch diet (ST) supplemented with 38.5% corn starch or a SUC containing 38.5% sucrose; the latter two diets were prepared by the addition of corn starch or sucrose, respectively, to CE‐2 (Table 1)"
Essentially they are diluting chow with starch or sucrose. Here is Table 1 for the diet compositions, note my red rectangle:
With group sizes of n=4 and five weeks on the diet very little of anything reached statistical or biological significance. The 2017 study used a slightly modified version of the diet to keep a low fat percentage identical across the diets but still had 38.5% of calories from sucrose, was run for 15 weeks and had group sizes of n=8-10. Results were statistically significant all over the place and suggest that the sucrose diet is decidedly good for metabolic health and gives a slim phenotype on ad lib consumption. Just so long as fat calories are very, very low. This looks very much like what Denise Minger described as carbosis, based in part around Walter Kempner's very effective, very unpleasant, ultra low fat, high sucrose medical diet. The Rice Diet is very real.
This post is not about any of the above.
Now, watch carefully. I'm going to sneak in some more macros
If you wanted a "reduced" fat diet which induces carbosis in human beings I recon the red text is pretty well it. I particularly enjoyed that exactly 7.7% of calories came from fat in each diet, this could almost be deliberate. If you combine what is almost certainly a very effective spontaneous weight loss diet with a 30% calorie restriction I suspect you might be on to a winner when comparing it against a reduced carbohydrate diet. Of course to really nail it you would have to compare it to an absolutely non ketogenic diet, say one supplying a total of 140g/d of carbohydrate. Does carbosis beat a middling carbohydrate mixed diet? You bet.
Oh, the scribbled-in red numbers came from Table 2 of this paper.
Most people in respectable CICO based mainstream nutrition have never heard of carbosis, Walter Kempner, the Rice Diet and have probably never heard of Denise Minger.
But Kevin Hall has. My respect for his knowledge-base and ingenuity is vast. Such a pity it's wasted on constructing props for his bizarre pet theories of weight control.
While the 7.7% of calories as fat in both studies is something which amuses me greatly, I do have to admit it may just be an hysterical accident.
At least I'm up front about my rather pronounced personal biases and rather peculiar sense of humour.
Peter
Chronic high-sucrose diet increases fibroblast growth factor 21 production and energy expenditure in mice
I've got a draft of a post from mid summer this year which I wrote simply because I like the attitude of the authors. They say things like:
"Excess carbohydrate intake causes obesity in humans".
That's the first line of the abstract. You know, it's that "nailing your colours to the mast" sort of a statement. Even though I do think life is a little more complex than that.
Anyway, I like these folks who are looking at the slimming effect of sucrose in BL6 mice. That's correct, sucrose is a slimming drug/food in mice, under the correct circumstances. People too. The data in the 2017 paper is an extension of the work they did in 2012, written up in this paper:
Ingestion of a moderate high‐sucrose diet results in glucose intolerance with reduced liver glucokinase activity and impaired glucagon‐like peptide‐1 secretion
I don't intend to go through either paper in detail, it's just that the 2012 paper has some rather special macro ratios that caught my eye.
This is what they did to the mice in that original paper:
"After adaptation for 2 weeks, they [the mice] were divided into three groups and fed a normal chow diet (NC), a high‐starch diet (ST) supplemented with 38.5% corn starch or a SUC containing 38.5% sucrose; the latter two diets were prepared by the addition of corn starch or sucrose, respectively, to CE‐2 (Table 1)"
Essentially they are diluting chow with starch or sucrose. Here is Table 1 for the diet compositions, note my red rectangle:
With group sizes of n=4 and five weeks on the diet very little of anything reached statistical or biological significance. The 2017 study used a slightly modified version of the diet to keep a low fat percentage identical across the diets but still had 38.5% of calories from sucrose, was run for 15 weeks and had group sizes of n=8-10. Results were statistically significant all over the place and suggest that the sucrose diet is decidedly good for metabolic health and gives a slim phenotype on ad lib consumption. Just so long as fat calories are very, very low. This looks very much like what Denise Minger described as carbosis, based in part around Walter Kempner's very effective, very unpleasant, ultra low fat, high sucrose medical diet. The Rice Diet is very real.
This post is not about any of the above.
Now, watch carefully. I'm going to sneak in some more macros
If you wanted a "reduced" fat diet which induces carbosis in human beings I recon the red text is pretty well it. I particularly enjoyed that exactly 7.7% of calories came from fat in each diet, this could almost be deliberate. If you combine what is almost certainly a very effective spontaneous weight loss diet with a 30% calorie restriction I suspect you might be on to a winner when comparing it against a reduced carbohydrate diet. Of course to really nail it you would have to compare it to an absolutely non ketogenic diet, say one supplying a total of 140g/d of carbohydrate. Does carbosis beat a middling carbohydrate mixed diet? You bet.
Oh, the scribbled-in red numbers came from Table 2 of this paper.
Most people in respectable CICO based mainstream nutrition have never heard of carbosis, Walter Kempner, the Rice Diet and have probably never heard of Denise Minger.
But Kevin Hall has. My respect for his knowledge-base and ingenuity is vast. Such a pity it's wasted on constructing props for his bizarre pet theories of weight control.
While the 7.7% of calories as fat in both studies is something which amuses me greatly, I do have to admit it may just be an hysterical accident.
At least I'm up front about my rather pronounced personal biases and rather peculiar sense of humour.
Peter
Tuesday, December 04, 2018
An exchange of half bricks
I would guess that everyone is aware of the study by Ebbeling et al, Ludwig's group, looking at the metabolic effect of low carbohydrate diets on total energy expenditure (TEE, all graphs show kcal/d) in the aftermath of weight loss on a conventional diet.
Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial
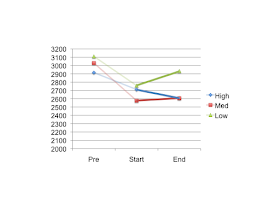
I'd like to summarise their data using numbers taken from Tables 2 and 3 which, with a little arithmetic, allows me to produce this graph of TEE at various time points. These are as follows: when the subjects walk off the street (Pre on the graph), after a period of semi-starvation on a conventional diet (Start) and then during weight stability on a high, medium or low carbohydrate diet (End). The plot looks like this:
In the study they compared the change from the Start TEE to the End TEE, ie they used these data points:
They took the absolute changes from Start to End thus and got a resultant p of less than 0.05
This, obviously, is completely unacceptable. Well, it is if you are Kevin Hall. So now we have this
No Significant Effect of Dietary Carbohydrate versus Fat on the Reduction in Total Energy Expenditure During Maintenance of Lost Weight
What Ludwig's group did wrong (amongst the many other things pointed out by Hall and Guo) is that they used the wrong data points.
Recall the original graph:
According to Hall: If you want to ask about the effect of low carbohydrate diets on the depression in TEE produced by conventional semi-starvation you should NOT compare the semi-starved TEE (as in Start) to the TEE on a high, medium or low carbohydrate diet (End). You should instead use the TEE expenditure at randomisation (Pre on the graph). Like this:
Using Pre as your anchor point you can draw the same data thus:
Which obviously gives us p greater than 0.05 and all of the benefits of low carbohydrate diets are lost. Phew. Happy Hall. But why should anyone use the Pre values as an anchor point?
Now, no one is an unbiased researcher. Hall is, surprisingly, no exception. Hence the current exchange of half bricks in the BMJ.
As I see it the Ebbeling paper looks at the effect of LC eating on the damage done to TEE by conventional dieting.
What Hall wants the analysis to do instead is to look at the overall effect of damage done to TEE by conventional semi-starvation combined with partial rescue during weight-stable LC eating vs the combined damage done by conventional semi-starvation followed by maintained damage done by HC weight-stable eating. As he writes:
"However, the final analysis plan was modified to make the diet comparisons with the TEE measurements collected in the immediate post-weight loss period rather than at the pre-weight loss baseline"
To me Hall is stating that Ebbeling et al almost did make the "Hall" mistake of using the "Pre" TTE as anchor point but corrected this at the 11th hour, still before blinding was unmasked. What puzzles me is how Ebbeling could have ever even considered using the "pre weight loss baseline" as the anchor point in the original study design.
The massive benefit to Hall of including the conventional semi-starvation active weight loss period along with the intervention weight stability period is to dilute the remedial biological effect of LC eating out of statistical significance.
The core information which the study provides is about the remedial effect of LC eating on correcting the damage done by a conventional semi-starvation period. That effect only happens between "Start" and "End", which is when carbohydrate restriction is applied.
That's one of the MASSIVE problems with carbohydrate restricted eating. It only provides benefit when you don't eat carbohydrate!
Including data from "Pre" right through to "End" dilutes the clearly demonstrable biological effect of carbohydrate restriction on reduced TEE post conventional dieting.
So what doe the title and text of Hall's rebuttal tell us? Either about Hall or about TEE? Don't over think it!
I would also declare that my own biases are a conflict of interest but if you need me to say that then you have probably arrived here by accident, you know where the back button is.
However I would say that I am ambivalent about the importance of the TEE changes, though I suspect they do happen. What really matters to me is what happened in Aberdeen over a decade ago.
Peter
Edit
More excellent half bricks here
End edit.
Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial
I'd like to summarise their data using numbers taken from Tables 2 and 3 which, with a little arithmetic, allows me to produce this graph of TEE at various time points. These are as follows: when the subjects walk off the street (Pre on the graph), after a period of semi-starvation on a conventional diet (Start) and then during weight stability on a high, medium or low carbohydrate diet (End). The plot looks like this:
In the study they compared the change from the Start TEE to the End TEE, ie they used these data points:
They took the absolute changes from Start to End thus and got a resultant p of less than 0.05
This, obviously, is completely unacceptable. Well, it is if you are Kevin Hall. So now we have this
No Significant Effect of Dietary Carbohydrate versus Fat on the Reduction in Total Energy Expenditure During Maintenance of Lost Weight
What Ludwig's group did wrong (amongst the many other things pointed out by Hall and Guo) is that they used the wrong data points.
Recall the original graph:
According to Hall: If you want to ask about the effect of low carbohydrate diets on the depression in TEE produced by conventional semi-starvation you should NOT compare the semi-starved TEE (as in Start) to the TEE on a high, medium or low carbohydrate diet (End). You should instead use the TEE expenditure at randomisation (Pre on the graph). Like this:
Using Pre as your anchor point you can draw the same data thus:
Which obviously gives us p greater than 0.05 and all of the benefits of low carbohydrate diets are lost. Phew. Happy Hall. But why should anyone use the Pre values as an anchor point?
Now, no one is an unbiased researcher. Hall is, surprisingly, no exception. Hence the current exchange of half bricks in the BMJ.
As I see it the Ebbeling paper looks at the effect of LC eating on the damage done to TEE by conventional dieting.
What Hall wants the analysis to do instead is to look at the overall effect of damage done to TEE by conventional semi-starvation combined with partial rescue during weight-stable LC eating vs the combined damage done by conventional semi-starvation followed by maintained damage done by HC weight-stable eating. As he writes:
"However, the final analysis plan was modified to make the diet comparisons with the TEE measurements collected in the immediate post-weight loss period rather than at the pre-weight loss baseline"
To me Hall is stating that Ebbeling et al almost did make the "Hall" mistake of using the "Pre" TTE as anchor point but corrected this at the 11th hour, still before blinding was unmasked. What puzzles me is how Ebbeling could have ever even considered using the "pre weight loss baseline" as the anchor point in the original study design.
The massive benefit to Hall of including the conventional semi-starvation active weight loss period along with the intervention weight stability period is to dilute the remedial biological effect of LC eating out of statistical significance.
The core information which the study provides is about the remedial effect of LC eating on correcting the damage done by a conventional semi-starvation period. That effect only happens between "Start" and "End", which is when carbohydrate restriction is applied.
That's one of the MASSIVE problems with carbohydrate restricted eating. It only provides benefit when you don't eat carbohydrate!
Including data from "Pre" right through to "End" dilutes the clearly demonstrable biological effect of carbohydrate restriction on reduced TEE post conventional dieting.
So what doe the title and text of Hall's rebuttal tell us? Either about Hall or about TEE? Don't over think it!
I would also declare that my own biases are a conflict of interest but if you need me to say that then you have probably arrived here by accident, you know where the back button is.
However I would say that I am ambivalent about the importance of the TEE changes, though I suspect they do happen. What really matters to me is what happened in Aberdeen over a decade ago.
Peter
Edit
More excellent half bricks here
End edit.
Sunday, November 25, 2018
More on insulin and the glycerophosphate shuttle
Raphi tweeted this paper recently
Nutritional Ketosis Increases NAD+/NADH Ratio in Healthy Human Brain: An in Vivo Study by 31P-MRS
which is nice provided, as he comments, it can be replicated. There is absolutely no possible conflict of interest anywhere so long as you accept it looks like an in-house Nestlé study. I haven't knowingly bought a Nestlé product in over 30 years.
Anyway. The study looks at healthy brain biochemistry under MCT induced ketosis. The ketone oxidation (or possibly the CNS oxidation of MCTs) increases the NAD+:NADH ratio, ie moves it in the Good direction.
There is a lot of talk about the NADH generation and NAD+ depletion during glycolysis to pyruvate, shifting the ratio in the Bad direction. The assumption (with which I disagree) is that the glycerophosphate shuttle is a rescue mechanism to regenerate essential NAD+ to allow glycolysis to continue, to which I will return in a moment.
The beauty of ketones is that they do not deplete cytoplasmic NAD+ at all and only consume one mitochondrial NAD+ during the conversion of BHB to AcAc. Because this happens within the mitochondria this, plus any NADH generated at the pyruvate dehydrogenase complex, is sitting next to complex I, the most prolific re-generator of NAD+ in the cell...
All well and good and bully for ketones and the manufacturers of Peptamen®1.5 Vanilla (Nestlé Health Science SA).
This got me thinking.
Of course no one in their right mind would expect glycolysis to be arranged in such a manner as to require the glycerophosphate shuttle for simple NAD+ regeneration. This is a wasteful loss of four pumped protons and this energy will appear as heat. Think of brown adipose tissue, full of mtG3Pdh, assuming insulin is plentiful. The correct pathway for the metabolism of glucose without insulin is to lactate without any overall depletion of cytoplasmic NAD+. Lactate can then be taken up by mitochondria exactly as ketones are. Lactate will, in the mitochondria, be reconverted to pyruvate, depleting mitochondrial NAD+ in exactly the same way as the conversion of BHB to AcAc does. Equally this happen right next door to complex I, just waiting to regenerate NAD+ and keep that NAD+:NADH ratio nice and high.
The whole point of the glycerophosphate shuttle (in Protons terms) is to facilitate insulin signalling. Insulin is the hormone of plenty, used to encourage caloric ingress in to cells. Loss of the four pumped protons due to bypassing complex I and using mtG3Pdh instead as part of insulin signalling appears perfectly reasonable under conditions of active caloric ingress. Sustained insulin signalling causes sustained loss of cytoplasmic NADH, which generates NAD+. Once this has happened there is no longer the surfeit of cytoplasmic NADH over NAD+ from glycolysis, which is essential to drive lactate formation. Glycolysis must therefor stop at pyruvate under insulin.
Summary: For insulin signalling the glycerophosphate shuttle is active and loss of NADH requires glycolysis to abort at pyruvate.
Without insulin signalling glycolysis runs to lactate which enters mitochondria without any depletion of cytoplasmic NAD+. The lactate should enter the mitochondria, under normal physiology.
Sooooooo. This had me thinking about what would happen if, in the presence of copious glucose and copious oxygen, there was to be a sudden profound fall in absolute insulin levels. I was particularly interested in systemic lactate levels.
A sudden, profound fall in insulin levels in the presence of glucose is pathology. It generates ketoacidosis, classically from acute beta cell destruction during the onset of DMT1. There is always a profound metabolic acidosis from the failure to suppress glucagon-induced lipolysis and subsequent massive acidic ketone generation. Under the canonical view the absence of insulin should not stop NAD+ regeneration by the glycerophosphate shuttle.
What I wanted to know was whether the Protons predicted shutting down of the glycerophosphate shuttle due to hypoinsulinaemia would result in diversion past pyruvate to lactate as the end result of glycolysis. In the presence of massive levels of ketones I would also expect this lactate to appear in the systemic situation.
Does it?
Yep. Ten seconds on Google says so.
Lactic acidosis in diabetic ketoacidosis
Very nice. I had no idea this was the case because it has no direct influence on treating DKA clinically...
Peter
Of course you have to think about the chicken and egg situation with insulin and mtG3Pdh activation (I have been for years!). Which comes first? I think insulin appears to be essential, as above. I do wonder if the insulin receptor will turn out to dock with the glycerophosphate shuttle in some way...
Nutritional Ketosis Increases NAD+/NADH Ratio in Healthy Human Brain: An in Vivo Study by 31P-MRS
which is nice provided, as he comments, it can be replicated. There is absolutely no possible conflict of interest anywhere so long as you accept it looks like an in-house Nestlé study. I haven't knowingly bought a Nestlé product in over 30 years.
Anyway. The study looks at healthy brain biochemistry under MCT induced ketosis. The ketone oxidation (or possibly the CNS oxidation of MCTs) increases the NAD+:NADH ratio, ie moves it in the Good direction.
There is a lot of talk about the NADH generation and NAD+ depletion during glycolysis to pyruvate, shifting the ratio in the Bad direction. The assumption (with which I disagree) is that the glycerophosphate shuttle is a rescue mechanism to regenerate essential NAD+ to allow glycolysis to continue, to which I will return in a moment.
The beauty of ketones is that they do not deplete cytoplasmic NAD+ at all and only consume one mitochondrial NAD+ during the conversion of BHB to AcAc. Because this happens within the mitochondria this, plus any NADH generated at the pyruvate dehydrogenase complex, is sitting next to complex I, the most prolific re-generator of NAD+ in the cell...
All well and good and bully for ketones and the manufacturers of Peptamen®1.5 Vanilla (Nestlé Health Science SA).
This got me thinking.
Of course no one in their right mind would expect glycolysis to be arranged in such a manner as to require the glycerophosphate shuttle for simple NAD+ regeneration. This is a wasteful loss of four pumped protons and this energy will appear as heat. Think of brown adipose tissue, full of mtG3Pdh, assuming insulin is plentiful. The correct pathway for the metabolism of glucose without insulin is to lactate without any overall depletion of cytoplasmic NAD+. Lactate can then be taken up by mitochondria exactly as ketones are. Lactate will, in the mitochondria, be reconverted to pyruvate, depleting mitochondrial NAD+ in exactly the same way as the conversion of BHB to AcAc does. Equally this happen right next door to complex I, just waiting to regenerate NAD+ and keep that NAD+:NADH ratio nice and high.
The whole point of the glycerophosphate shuttle (in Protons terms) is to facilitate insulin signalling. Insulin is the hormone of plenty, used to encourage caloric ingress in to cells. Loss of the four pumped protons due to bypassing complex I and using mtG3Pdh instead as part of insulin signalling appears perfectly reasonable under conditions of active caloric ingress. Sustained insulin signalling causes sustained loss of cytoplasmic NADH, which generates NAD+. Once this has happened there is no longer the surfeit of cytoplasmic NADH over NAD+ from glycolysis, which is essential to drive lactate formation. Glycolysis must therefor stop at pyruvate under insulin.
Summary: For insulin signalling the glycerophosphate shuttle is active and loss of NADH requires glycolysis to abort at pyruvate.
Without insulin signalling glycolysis runs to lactate which enters mitochondria without any depletion of cytoplasmic NAD+. The lactate should enter the mitochondria, under normal physiology.
Sooooooo. This had me thinking about what would happen if, in the presence of copious glucose and copious oxygen, there was to be a sudden profound fall in absolute insulin levels. I was particularly interested in systemic lactate levels.
A sudden, profound fall in insulin levels in the presence of glucose is pathology. It generates ketoacidosis, classically from acute beta cell destruction during the onset of DMT1. There is always a profound metabolic acidosis from the failure to suppress glucagon-induced lipolysis and subsequent massive acidic ketone generation. Under the canonical view the absence of insulin should not stop NAD+ regeneration by the glycerophosphate shuttle.
What I wanted to know was whether the Protons predicted shutting down of the glycerophosphate shuttle due to hypoinsulinaemia would result in diversion past pyruvate to lactate as the end result of glycolysis. In the presence of massive levels of ketones I would also expect this lactate to appear in the systemic situation.
Does it?
Yep. Ten seconds on Google says so.
Lactic acidosis in diabetic ketoacidosis
Very nice. I had no idea this was the case because it has no direct influence on treating DKA clinically...
Peter
Of course you have to think about the chicken and egg situation with insulin and mtG3Pdh activation (I have been for years!). Which comes first? I think insulin appears to be essential, as above. I do wonder if the insulin receptor will turn out to dock with the glycerophosphate shuttle in some way...
Saturday, November 24, 2018
Metallic iron and the origin of metabolism
Over the years I've been convinced that carbon monoxide derived formaldehyde/formate are probably the initial molecular precursors of acetate at the origin of life. All that is needed is a supply of electrons at a sufficiently negative potential to reduce CO2 to CO and so to CH2O then to HCOOH, formate. Clearly a 1.5 volt battery applied across an anoxic CO2 rich reactor might do this. In the Life series of posts the best candidate in reality is the alkaline hydrothermal vent environment such as the Lost City complex, working under anoxic, CO2 rich Hadean ocean conditions.
This paper:
Native iron reduces CO2 to intermediates and endproducts of the acetyl-CoA pathway
from a french institute, suggests that metallic iron alone might provide electrons of sufficiently negative potential to perform the process, this is the basic premise:
Fe0 → Fe2++ 2e-
These electrons have a sufficiently negative potential to allow:
CO2 + 2e- + H2O → HCOOH + O2-
Obviously the Fe2+ would combine with the O2- to give FeO, leaving a formate moiety as the start of the process essential for the origin of pre-biotic metabolism.
In the event the two most common experimental products were acetate and pyruvate, a highly plausible step or two onward from formate, which they also found under certain conditions.
The circumstances of temperature and pressure were, in some experiments, plausible for pre-biotic chemistry.
The problems, compared to the Lane and Martin hydrothermal vents concept, seem to be:
The products are bound to the surface of the iron deposit, potassium hydroxide was needed to hydrolyse them off for measurement.
The process is reactive rather than catalytic, ie the metallic iron is consumed in the process of providing electrons. This contrasts starkly with the continuous supply of electrons supplied by hydrothermal vent conditions over geological time scales.
Then there is the concentration problem. If the organic products were to be freed from the iron surface they need to be somewhere other than the open deep ocean or they will simply be lost by dilution.
Finally the group did not cite any of the work from Nick Lane and his lab excepting one rather general review link. Naughty.
So. Some interesting chemistry and it's good to have multiple groups thinking about a given problem but I don't see the hydrothermal vent hypothesis being abandoned any time soon. Certainly not by believers like myself.
Peter
This paper:
Native iron reduces CO2 to intermediates and endproducts of the acetyl-CoA pathway
from a french institute, suggests that metallic iron alone might provide electrons of sufficiently negative potential to perform the process, this is the basic premise:
Fe0 → Fe2++ 2e-
These electrons have a sufficiently negative potential to allow:
CO2 + 2e- + H2O → HCOOH + O2-
Obviously the Fe2+ would combine with the O2- to give FeO, leaving a formate moiety as the start of the process essential for the origin of pre-biotic metabolism.
In the event the two most common experimental products were acetate and pyruvate, a highly plausible step or two onward from formate, which they also found under certain conditions.
The circumstances of temperature and pressure were, in some experiments, plausible for pre-biotic chemistry.
The problems, compared to the Lane and Martin hydrothermal vents concept, seem to be:
The products are bound to the surface of the iron deposit, potassium hydroxide was needed to hydrolyse them off for measurement.
The process is reactive rather than catalytic, ie the metallic iron is consumed in the process of providing electrons. This contrasts starkly with the continuous supply of electrons supplied by hydrothermal vent conditions over geological time scales.
Then there is the concentration problem. If the organic products were to be freed from the iron surface they need to be somewhere other than the open deep ocean or they will simply be lost by dilution.
Finally the group did not cite any of the work from Nick Lane and his lab excepting one rather general review link. Naughty.
So. Some interesting chemistry and it's good to have multiple groups thinking about a given problem but I don't see the hydrothermal vent hypothesis being abandoned any time soon. Certainly not by believers like myself.
Peter
Wednesday, November 14, 2018
A brief aside in to statins and FH and all cause mortality
I must admit that I have not read this paper, just the abstract. My excuse is, once again, that I have no access to any ondansetron.
Statins in Familial Hypercholesterolemia: Consequences for Coronary Artery Disease and All-Cause Mortality
As always the results of statin therapy are, to say the least, dramatic.
"In patients with heterozygous FH, moderate- to high-intensity statin therapy lowered the risk for CAD and mortality by 44%".
Wow. But why the need for a composite end point?
If we leave aside soft end points which include coronary re-vascularisation (never influenced by serum lipid levels. No laughing at the back there!) and concentrate on the hard end point of all cause mortality we end up with, for non statinated people:
9 deaths per 4,892 person-years, which I make 1.8 deaths per 1000 person-years.
On a statin we have 17 deaths per 11,674 person-years, 1.5 per 1000 person-years.
That looks like a reduction in mortality of 0.3 people per 1000 person-years.
Or, being more whole numberish, 1 person saved by treating for 3,300 person-years on a statin.
Does that convert to treating 100 people for 33 years to avoid one premature fatality? We're all going to die one day so no one avoids death permanently, even by taking a statin. Unbelievable as that sounds.
If you have heterozygous FH your chances of dying tomorrow are rather low but not quite zero. If you take a statin it will reduce this chance by a vanishingly small amount.
Taking the difference between "rather-low-but-not-quite-zero" and "a-vanishingly-small-amount less than rather-low-but-not-quite-zero", dividing this difference by "rather-low-but-not-quite-zero" and multiplying by 100 we get a massive 17% reduction in all cause mortality. Which means diddly squat, but sounds good if you are a statinator. Admittedly not as good as 44% for the composite end point but hey... Neither means anything.
The main benefit of a statin appears to be that the number it gives you on a lab report might just influence a cardiologist to leave your coronary arteries alone.
Peter
Statins in Familial Hypercholesterolemia: Consequences for Coronary Artery Disease and All-Cause Mortality
As always the results of statin therapy are, to say the least, dramatic.
"In patients with heterozygous FH, moderate- to high-intensity statin therapy lowered the risk for CAD and mortality by 44%".
Wow. But why the need for a composite end point?
If we leave aside soft end points which include coronary re-vascularisation (never influenced by serum lipid levels. No laughing at the back there!) and concentrate on the hard end point of all cause mortality we end up with, for non statinated people:
9 deaths per 4,892 person-years, which I make 1.8 deaths per 1000 person-years.
On a statin we have 17 deaths per 11,674 person-years, 1.5 per 1000 person-years.
That looks like a reduction in mortality of 0.3 people per 1000 person-years.
Or, being more whole numberish, 1 person saved by treating for 3,300 person-years on a statin.
Does that convert to treating 100 people for 33 years to avoid one premature fatality? We're all going to die one day so no one avoids death permanently, even by taking a statin. Unbelievable as that sounds.
If you have heterozygous FH your chances of dying tomorrow are rather low but not quite zero. If you take a statin it will reduce this chance by a vanishingly small amount.
Taking the difference between "rather-low-but-not-quite-zero" and "a-vanishingly-small-amount less than rather-low-but-not-quite-zero", dividing this difference by "rather-low-but-not-quite-zero" and multiplying by 100 we get a massive 17% reduction in all cause mortality. Which means diddly squat, but sounds good if you are a statinator. Admittedly not as good as 44% for the composite end point but hey... Neither means anything.
The main benefit of a statin appears to be that the number it gives you on a lab report might just influence a cardiologist to leave your coronary arteries alone.
Peter
Listeriosis is no fun
Just doing my bit
Vegetables, nine dead of listeriosis
Quick edit for when the link dies:
"9 people dead following Listeria outbreak – Tesco, Aldi, Waitrose, Iceland, Lidl, Aldi – Issue Product recall. Please please check on old people and loved ones who may not be in the loop, listeria can be more serious for people who have weakened immune systems.
Full 43 product list for recall is shown as follows issued by the FSA".
All vegetables.
Peter
Vegetables, nine dead of listeriosis
Quick edit for when the link dies:
"9 people dead following Listeria outbreak – Tesco, Aldi, Waitrose, Iceland, Lidl, Aldi – Issue Product recall. Please please check on old people and loved ones who may not be in the loop, listeria can be more serious for people who have weakened immune systems.
Full 43 product list for recall is shown as follows issued by the FSA".
All vegetables.
Peter
Wednesday, November 07, 2018
Green Tea Extract; superb antioxidant?
Here is a little more from this paper:
High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS
This is insulin signalling under massively supra-physiological insulin exposure in cell culture:
This is, obviously, their best gel, that's the one you publish. The insulin resistance (fainter P-Akt band) when insulin and Se are both used compared to insulin w/o Se exposure does appear to be there. At physiological levels of insulin this differential seems likely to be maintained.
This implies blunting of insulin signalling, which allows more FFA oxidation, which generates greater levels of ROS than would occur under continued insulin action. These ROS would be physiological on a ketogenic diet or under extended fasting but are not so in cells under culture using 11mmol glucose (which is what I think is in the medium they used, they don't actually say) plus whatever insulin is present in 10% FBS. So we have this:
Control is from cells under RPMI 1640 alone, traditionally 11mmol/l glucose. Excess selenium blunts insulin signalling so allows FFA release from intracellular triglyceride stores, so increases ROS (in the same way as metformin does but w/o the suppression of gluconeogenesis intrinsic to metformin's action). Adding rotenone, as you would expect, blocks RET so blocks ROS generation. CCCP uncouples respiration, drops delta psi so blocks RET/ROS. Etomoxir blocks access of FFAs to mitochondria so blocks input at mtETFdh, so blocks RET/ROS. MitoQ powerfully targets all mitochondrial ROS so over-rides the FFA oxidation ROS generation effect. Chromium picolinate restores insulin signalling by repleting the Cr depletion induced by Se. MSA is an inhibitor of glutathione peroxidase, so it eliminates the effects of excess GPX. And SS, sodium salicylate, appears to block intracellular lipolysis in hepatocytes, so suppresses fatty acid supply to mitochondria, much as insulin or etomoxir would.
All a very plausible narrative.
Except for oligomycin. What does anyone expect the blockade of ATP synthase to do to ROS generation, throughout the electron transport chain? It is going to increase delta psi, reduce all of the redox complexes and generate a ton of RET and ROS through complex I and probably at ton at complex III too. It is specifically used to generate ROS in many other studies, example here:
The specificity of neuroprotection by antioxidants
I'm not very comfortable with oligomycin as a suppressor of FFA oxidation induced ROS. It is another, rather serious, blight on the paper. It certainly should have been discussed.
I would usually ignore the whole paper except Tom Naughton gave us all the heads up on a recent report of a chap taking what might have been a hefty dose of green tea extract who went in to liver failure. Obviously most folks just excrete antioxidants like GTE with little harm done. I just wonder if he got unlucky or took a huge dose while walking round with the sort of liver full of lipid so beloved of Public Health England. Losing the protection of insulin's inhibition of lipolysis simply dumped a ton of unregulated intra-hepatocyte FFAs from lipid droplets on to his mitochondria, which then popped their clogs.
Who knows? It's another nice narrative. I just wish I wasn't so suspicious of the selenium paper...
Both reports also play rather too well to my biases against antioxidants, but that's how it is...
Peter
High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS
This is insulin signalling under massively supra-physiological insulin exposure in cell culture:
This is, obviously, their best gel, that's the one you publish. The insulin resistance (fainter P-Akt band) when insulin and Se are both used compared to insulin w/o Se exposure does appear to be there. At physiological levels of insulin this differential seems likely to be maintained.
This implies blunting of insulin signalling, which allows more FFA oxidation, which generates greater levels of ROS than would occur under continued insulin action. These ROS would be physiological on a ketogenic diet or under extended fasting but are not so in cells under culture using 11mmol glucose (which is what I think is in the medium they used, they don't actually say) plus whatever insulin is present in 10% FBS. So we have this:
Control is from cells under RPMI 1640 alone, traditionally 11mmol/l glucose. Excess selenium blunts insulin signalling so allows FFA release from intracellular triglyceride stores, so increases ROS (in the same way as metformin does but w/o the suppression of gluconeogenesis intrinsic to metformin's action). Adding rotenone, as you would expect, blocks RET so blocks ROS generation. CCCP uncouples respiration, drops delta psi so blocks RET/ROS. Etomoxir blocks access of FFAs to mitochondria so blocks input at mtETFdh, so blocks RET/ROS. MitoQ powerfully targets all mitochondrial ROS so over-rides the FFA oxidation ROS generation effect. Chromium picolinate restores insulin signalling by repleting the Cr depletion induced by Se. MSA is an inhibitor of glutathione peroxidase, so it eliminates the effects of excess GPX. And SS, sodium salicylate, appears to block intracellular lipolysis in hepatocytes, so suppresses fatty acid supply to mitochondria, much as insulin or etomoxir would.
All a very plausible narrative.
Except for oligomycin. What does anyone expect the blockade of ATP synthase to do to ROS generation, throughout the electron transport chain? It is going to increase delta psi, reduce all of the redox complexes and generate a ton of RET and ROS through complex I and probably at ton at complex III too. It is specifically used to generate ROS in many other studies, example here:
The specificity of neuroprotection by antioxidants
I'm not very comfortable with oligomycin as a suppressor of FFA oxidation induced ROS. It is another, rather serious, blight on the paper. It certainly should have been discussed.
I would usually ignore the whole paper except Tom Naughton gave us all the heads up on a recent report of a chap taking what might have been a hefty dose of green tea extract who went in to liver failure. Obviously most folks just excrete antioxidants like GTE with little harm done. I just wonder if he got unlucky or took a huge dose while walking round with the sort of liver full of lipid so beloved of Public Health England. Losing the protection of insulin's inhibition of lipolysis simply dumped a ton of unregulated intra-hepatocyte FFAs from lipid droplets on to his mitochondria, which then popped their clogs.
Who knows? It's another nice narrative. I just wish I wasn't so suspicious of the selenium paper...
Both reports also play rather too well to my biases against antioxidants, but that's how it is...
Peter
Friday, November 02, 2018
Stone Agers in the Fast Lane?
A destruction of Paleo Diet™ as a management tool for metabolic syndrome in modern humans surfaced recently in a tweet from Miki Ben-Dor, along with his comment that he views meat as the default paleo food.
Plants used as "food" come and go and are nowadays developed in to reduced toxicity versions which are what we call vegetables. Meat is meat and even the invention of factory farming does not seem to be able to convert it in to anything as toxic as a courgette. Remember this?
Courgette stew kills pensioner in Heidelberg
Anyway, back to the Pacific Islanders. This is the book chapter we're interested in and it's entertaining.
Stone Agers in the Fast Lane? How Bioarchaeologists Can Address the Paleo Diet Myth
These people appear to have read (and cite) essentially every paper on gout in archeological record of the paleo Pacific Islander population. They are using gout as a skeletally preserved marker for metabolic syndrome, a fairly reasonable approach to my mind. The assumption that meat causes gout (lots of purines don'tchano) which is threaded throughout the chapter is less acceptable.
So we end up with this as a core summing up close to the end, for anyone who doesn't want to slog through the various straw men they set up to knock down:
"We have also used a case study of Pacific Islanders’ experiences with MetS and paleopathology evidence of gout to reexamine the very basis for the “necessity” of a return to a Paleo Diet. As discussed, the ancestral diet (based on tuberous root crops, not cereals) and population history of Pacific islanders are completely different to the Old and New Worlds where the Paleo Diet debate is entrenched. Yet the burden of MetS is extremely high in the Pacific. While the adoption of westernized diets has exacerbated the expression of MetS conditions in modern Polynesians, the paleopathological evidence (especially gout) suggests the origins of these conditions stems from their Lapita ancestors, who in turn trace their roots back to Island Southeast Asia".
Gout was widespread in the pre-Westernisation Pacific Islanders, despite their paleo diet. The core quote re this paleo diet is that it is "based on tuberous root crops, not cereals".
Translation: A Paleo Diet diet based on paleo tuberous root crops gives you paleo gout.
Eat some meat and get your calories from fat. Vegetables can be viewed as a recreational indulgence if you so wish. But maybe don't over do them unless you want Paleo Diet™ gout.
Peter
Plants used as "food" come and go and are nowadays developed in to reduced toxicity versions which are what we call vegetables. Meat is meat and even the invention of factory farming does not seem to be able to convert it in to anything as toxic as a courgette. Remember this?
Courgette stew kills pensioner in Heidelberg
Anyway, back to the Pacific Islanders. This is the book chapter we're interested in and it's entertaining.
Stone Agers in the Fast Lane? How Bioarchaeologists Can Address the Paleo Diet Myth
These people appear to have read (and cite) essentially every paper on gout in archeological record of the paleo Pacific Islander population. They are using gout as a skeletally preserved marker for metabolic syndrome, a fairly reasonable approach to my mind. The assumption that meat causes gout (lots of purines don'tchano) which is threaded throughout the chapter is less acceptable.
So we end up with this as a core summing up close to the end, for anyone who doesn't want to slog through the various straw men they set up to knock down:
"We have also used a case study of Pacific Islanders’ experiences with MetS and paleopathology evidence of gout to reexamine the very basis for the “necessity” of a return to a Paleo Diet. As discussed, the ancestral diet (based on tuberous root crops, not cereals) and population history of Pacific islanders are completely different to the Old and New Worlds where the Paleo Diet debate is entrenched. Yet the burden of MetS is extremely high in the Pacific. While the adoption of westernized diets has exacerbated the expression of MetS conditions in modern Polynesians, the paleopathological evidence (especially gout) suggests the origins of these conditions stems from their Lapita ancestors, who in turn trace their roots back to Island Southeast Asia".
Gout was widespread in the pre-Westernisation Pacific Islanders, despite their paleo diet. The core quote re this paleo diet is that it is "based on tuberous root crops, not cereals".
Translation: A Paleo Diet diet based on paleo tuberous root crops gives you paleo gout.
Eat some meat and get your calories from fat. Vegetables can be viewed as a recreational indulgence if you so wish. But maybe don't over do them unless you want Paleo Diet™ gout.
Peter
Tuesday, October 30, 2018
Selenium induced glutathione peroxidase generation
TLDR: excess selenium induces excess glutathione peroxidase which blunts physiological superoxide/H2O2 signalling within a cell. This, as you might expect, is a Bad Thing.
I stumbled across this paper quite by accident. It has a number of problems, not least of which is that none of the authors appears to be a native english speaker and this tends to show through. It also carries a rather catastrophic typo/brain fart in the abstract, where TCA, as in tricarboxylic acid cycle, was written out in full as trichloroacetic acid cycle. Ouch. Never the less, they don't seem to like antioxidants.
High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS
Over all they don't seem to like suppressing the physiological levels of ROS needed for insulin signalling, certainly while feeding their rats a high carbohydrate diet. Not that they tell us what they fed the rats on!
This is the intraperitoneal glucose tolerance test result, look at "control" and "Se" for the effect of the high selenium diet:
Among many of the problems with the paper another is the difficulty distinguishing which line is which on the graphs. I think we can say the top line is clearly the selenium supplemented group and the bottom line is probably the control group. The main effect is at the 30 minute mark. This suggests to me that there is an inadequate first phase insulin response (needing ROS from RET induced by mtG3Pdh) to overcome the systemic failure of insulin action (also needing ROS from RET via mtG3Pdh to diffuse as far as the insulin receptor). By 60 minutes the difference is negligible. Had they measured insulin I'd bet the second phase response was exaggerated.
Here is the intraperitoneal insulin tolerance test result:
This time the bottom line is clearly the control group, top line the selenium treated group. From the control group I see no suggestion that this particular dose of insulin is inducing insulin-induced insulin resistance, so all we see is the failure of the insulin signalling activating action of ROS at the two hour mark. Note the lines are all parallel until the one hour mark. This is what suggests that there is nothing wrong with insulin action per se at the 30 minute mark and is another factor which makes me suspect that there is a failure to secrete insulin during the early stages of the IPGTT. The normal response to exogenous insulin, up until one hour mark, occurs while ever the exogenous insulin can overcome the loss of ROS caused by the glutathione peroxidase excess induced by the high selenium diet.
As humans we deal with huge numbers of xenobiotic antioxidants, mostly from plants. For the vast majority we can simply use our liver and/or kidneys to dump them to where they can do us no harm. Just occasionally we fail, as with selenium. The end result is not pretty.
Peter
There are some more interesting finings in the paper which might be worth chatting about. Maybe another day.
Random throw-away thought: Is this how uric acid induces insulin resistance? By being too good an antioxidant???? Hmmmmmm.
I stumbled across this paper quite by accident. It has a number of problems, not least of which is that none of the authors appears to be a native english speaker and this tends to show through. It also carries a rather catastrophic typo/brain fart in the abstract, where TCA, as in tricarboxylic acid cycle, was written out in full as trichloroacetic acid cycle. Ouch. Never the less, they don't seem to like antioxidants.
High selenium impairs hepatic insulin sensitivity through opposite regulation of ROS
Over all they don't seem to like suppressing the physiological levels of ROS needed for insulin signalling, certainly while feeding their rats a high carbohydrate diet. Not that they tell us what they fed the rats on!
This is the intraperitoneal glucose tolerance test result, look at "control" and "Se" for the effect of the high selenium diet:
Among many of the problems with the paper another is the difficulty distinguishing which line is which on the graphs. I think we can say the top line is clearly the selenium supplemented group and the bottom line is probably the control group. The main effect is at the 30 minute mark. This suggests to me that there is an inadequate first phase insulin response (needing ROS from RET induced by mtG3Pdh) to overcome the systemic failure of insulin action (also needing ROS from RET via mtG3Pdh to diffuse as far as the insulin receptor). By 60 minutes the difference is negligible. Had they measured insulin I'd bet the second phase response was exaggerated.
Here is the intraperitoneal insulin tolerance test result:
This time the bottom line is clearly the control group, top line the selenium treated group. From the control group I see no suggestion that this particular dose of insulin is inducing insulin-induced insulin resistance, so all we see is the failure of the insulin signalling activating action of ROS at the two hour mark. Note the lines are all parallel until the one hour mark. This is what suggests that there is nothing wrong with insulin action per se at the 30 minute mark and is another factor which makes me suspect that there is a failure to secrete insulin during the early stages of the IPGTT. The normal response to exogenous insulin, up until one hour mark, occurs while ever the exogenous insulin can overcome the loss of ROS caused by the glutathione peroxidase excess induced by the high selenium diet.
As humans we deal with huge numbers of xenobiotic antioxidants, mostly from plants. For the vast majority we can simply use our liver and/or kidneys to dump them to where they can do us no harm. Just occasionally we fail, as with selenium. The end result is not pretty.
Peter
There are some more interesting finings in the paper which might be worth chatting about. Maybe another day.
Random throw-away thought: Is this how uric acid induces insulin resistance? By being too good an antioxidant???? Hmmmmmm.
Sunday, October 21, 2018
Metformin (09) Islets
This is another very abstracted study using isolated mouse islets in cell culture to assess the effect of metformin on insulin secretion.
Metformin Inhibits Mouse Islet Insulin Secretion and Alters Intracellular Calcium in a Concentration-Dependent and Duration-Dependent Manner near the Circulating Range
From the Protons perspective the factors which drive insulin signalling are the same ones which drive insulin secretion, certainly at low physiological concentrations. The situation is different under post prandial conditions where, eventually, reverse electron transport increases from low, physiological activating levels of ROS to the high physiological levels which drive insulin resistance rather than activation. Recall this is what I consider to be the cellular repletion signal, the one so easily mistaken for insulin as an anorexic agent. Anyway, here we have metformin acting under 11mmol/l of glucose to suppress insulin secretion.
Just to recap; 20micromolar metformin is therapeutic, 200micromolar is life threatening lactic acidosis and 1mmolar (1000micromolar) is death:
Any findings in the paper using concentrations of 200micromolar or higher can safely be ignored for therapeutic relevance. Except for the confirmation that cells die rather well at 1.0mmol of metformin and are doing rather more apoptosis than you might like at 200micromolar (see Figure 3 in the paper). No surprises there.
Also consider that picking up subtleties of insulin secretion by measuring the concentration in a culture well is a very blunt instrument. But at least they are looking.
So why doesn't metformin cause diabetes on the sort of criminal (up until very recently) diet advised by any diabetologist?
This is partly because the redox changes in the liver suppress gluconeogenesis, though the exact mechanism by which blockade of mtG3Pdh suppresses hepatic glucose production is debatable.
It's also because, certainly in peripheral cells suffering from chronic hyperinsulinaemia-induced lipotoxicity, cessation or reduction of insulin signalling will allow release of fatty acids able to generate their own RET via the oxidation of beta oxidation derived electron transporting flavoprotein at mtETFdh of the electron transport chain and so restore insulin signalling. Or, if there is enough superoxide, insulin resistance. So cells suddenly realise they have a great supply of FFAs, adequate ATP generation and no need for any more caloric ingress. Which generalises to the whole organism as a "no need to eat" state, which might just give weight loss. As metformin does.
Kind of like LC eating in a pill.
Peter
Actually metformin might do the same to lipid in the pancreas as it does in peripheral tissues. Loss of accumulated pancreatic lipid is what people like Dr Roy Taylor consider the mechanism by which the hypoinsulinaemia of semi-starvation induces some degree of remission of diabetes, in a few patients, while they can stick to it.
Metformin Inhibits Mouse Islet Insulin Secretion and Alters Intracellular Calcium in a Concentration-Dependent and Duration-Dependent Manner near the Circulating Range
From the Protons perspective the factors which drive insulin signalling are the same ones which drive insulin secretion, certainly at low physiological concentrations. The situation is different under post prandial conditions where, eventually, reverse electron transport increases from low, physiological activating levels of ROS to the high physiological levels which drive insulin resistance rather than activation. Recall this is what I consider to be the cellular repletion signal, the one so easily mistaken for insulin as an anorexic agent. Anyway, here we have metformin acting under 11mmol/l of glucose to suppress insulin secretion.
Just to recap; 20micromolar metformin is therapeutic, 200micromolar is life threatening lactic acidosis and 1mmolar (1000micromolar) is death:
Any findings in the paper using concentrations of 200micromolar or higher can safely be ignored for therapeutic relevance. Except for the confirmation that cells die rather well at 1.0mmol of metformin and are doing rather more apoptosis than you might like at 200micromolar (see Figure 3 in the paper). No surprises there.
Also consider that picking up subtleties of insulin secretion by measuring the concentration in a culture well is a very blunt instrument. But at least they are looking.
So why doesn't metformin cause diabetes on the sort of criminal (up until very recently) diet advised by any diabetologist?
This is partly because the redox changes in the liver suppress gluconeogenesis, though the exact mechanism by which blockade of mtG3Pdh suppresses hepatic glucose production is debatable.
It's also because, certainly in peripheral cells suffering from chronic hyperinsulinaemia-induced lipotoxicity, cessation or reduction of insulin signalling will allow release of fatty acids able to generate their own RET via the oxidation of beta oxidation derived electron transporting flavoprotein at mtETFdh of the electron transport chain and so restore insulin signalling. Or, if there is enough superoxide, insulin resistance. So cells suddenly realise they have a great supply of FFAs, adequate ATP generation and no need for any more caloric ingress. Which generalises to the whole organism as a "no need to eat" state, which might just give weight loss. As metformin does.
Kind of like LC eating in a pill.
Peter
Actually metformin might do the same to lipid in the pancreas as it does in peripheral tissues. Loss of accumulated pancreatic lipid is what people like Dr Roy Taylor consider the mechanism by which the hypoinsulinaemia of semi-starvation induces some degree of remission of diabetes, in a few patients, while they can stick to it.
Monday, October 15, 2018
Metformin (08) Insulin and AMPK
This is a simplified diagram of most of the pathways around AMP-kinase (AMKP) which I found at the website of a commercial company on tinternet
Obviously most of the diagram shows the Good Things which activating AMPK does. There appear to be three core activating signals; changes in Ca2+ via calmodulin, increasing cyclic AMP and that change in AMP+ADP:ATP triggered by exercise, low glucose, hypoxia and severely toxic doses of metformin. Here they are emphasised in blue:
There is only one inhibitory input shown, I've marked it in red:
When you follow this input back you end up with the one factor which suppresses AMPK signalling.
We know that metformin inhibits mtG3Pdh at normal pharmacological concentrations. From the Protons perspective this blocks the generation of the ROS essential for insulin signaling. No insulin signalling, no inhibition of AMPK. Which nicely fits in with this paper:
Insulin inhibits AMPK activity and phosphorylates AMPK Ser485/491 through Akt in hepatocytes, myotubes and incubated rat skeletal muscle
Of course metformin inhibits complex I (which drops ATP and so activates AMPK) at concentrations which put you in to the ITU with lactic acidosis, around 200micromolar in plasma. Recall this?
It also activates AMPK via inhibition of AMP-deaminase using tissue culture exposure of 10mmolar. That's 10,000micromolar, which would probably put you in to the morgue rather than the ITU.
A quick reality check suggests that taking one 500mg metformin tablet an hour before a bike race might just help you win... Somehow I don't think blockade of complex I would do that! Freeing up fatty acids by dumping insulin signalling might just do the job.
Peter
Obviously most of the diagram shows the Good Things which activating AMPK does. There appear to be three core activating signals; changes in Ca2+ via calmodulin, increasing cyclic AMP and that change in AMP+ADP:ATP triggered by exercise, low glucose, hypoxia and severely toxic doses of metformin. Here they are emphasised in blue:
There is only one inhibitory input shown, I've marked it in red:
When you follow this input back you end up with the one factor which suppresses AMPK signalling.
Insulin.
We know that metformin inhibits mtG3Pdh at normal pharmacological concentrations. From the Protons perspective this blocks the generation of the ROS essential for insulin signaling. No insulin signalling, no inhibition of AMPK. Which nicely fits in with this paper:
Insulin inhibits AMPK activity and phosphorylates AMPK Ser485/491 through Akt in hepatocytes, myotubes and incubated rat skeletal muscle
Of course metformin inhibits complex I (which drops ATP and so activates AMPK) at concentrations which put you in to the ITU with lactic acidosis, around 200micromolar in plasma. Recall this?
It also activates AMPK via inhibition of AMP-deaminase using tissue culture exposure of 10mmolar. That's 10,000micromolar, which would probably put you in to the morgue rather than the ITU.
A quick reality check suggests that taking one 500mg metformin tablet an hour before a bike race might just help you win... Somehow I don't think blockade of complex I would do that! Freeing up fatty acids by dumping insulin signalling might just do the job.
Peter
Saturday, September 15, 2018
Insulin makes you hungry (9) Women
Intranasal insulin does not, under any circumstances, reduce food intake or bodyweight in women.
That's half the population.
Hallschmid's lab gives us all of these:
Intranasal insulin reduces body fat in men but not in women
Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin
Comparable sensitivity of postmenopausal and young women to the effects of intranasal insulin on food intake and working memory
So. What about this via Wooo:
Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women
It's free full text if anyone want's to read the details of the convoluted protocol involved. Take my word, you will regret it. Here's the executive summary
You can summarise the results by saying intranasal insulin puts women off of Chocolate Chip Cookies. At least those made by Coppenrath of Geest in Germany. Maybe this will happen with chocolate Hobnobs in the UK or some sort of Oreo in the USA. Who knows? The subjects just filled up on the Premium Spritz Cookies and the Crunchy Coconut Cookies, all made by the same firm. There are lots of soft end points with low p values in the study. Hard end point to me is calories.
Does intranasal insulin affect total calorie intake of cookies, even in the convoluted protocol used?
No.
I have no idea why intranasal insulin is ineffective to speed cellular repletion in 50% of the world population but it makes me suspicious that women deal with calories slightly differently to men and that we're not going to find out how or why from squirting insulin up anyone's nose. And that insulin is not a satiety hormone.
I think I need to get outside more.
Peter
That's half the population.
Hallschmid's lab gives us all of these:
Intranasal insulin reduces body fat in men but not in women
Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin
Comparable sensitivity of postmenopausal and young women to the effects of intranasal insulin on food intake and working memory
So. What about this via Wooo:
Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women
It's free full text if anyone want's to read the details of the convoluted protocol involved. Take my word, you will regret it. Here's the executive summary
You can summarise the results by saying intranasal insulin puts women off of Chocolate Chip Cookies. At least those made by Coppenrath of Geest in Germany. Maybe this will happen with chocolate Hobnobs in the UK or some sort of Oreo in the USA. Who knows? The subjects just filled up on the Premium Spritz Cookies and the Crunchy Coconut Cookies, all made by the same firm. There are lots of soft end points with low p values in the study. Hard end point to me is calories.
Does intranasal insulin affect total calorie intake of cookies, even in the convoluted protocol used?
No.
I have no idea why intranasal insulin is ineffective to speed cellular repletion in 50% of the world population but it makes me suspicious that women deal with calories slightly differently to men and that we're not going to find out how or why from squirting insulin up anyone's nose. And that insulin is not a satiety hormone.
I think I need to get outside more.
Peter
Wednesday, September 12, 2018
Insulin makes you hungry (8) Blokes
Now it's time to look at the simple situation of men given intranasal insulin. We have a chronic study using 40iu and an acute study using 160iu.
Edit. Oops, forgot the links!
Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man
Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin
End edit.
I've not found an acute study using 40iu but we can be pretty sure it works in the same manner as the acute 160iu dose. The only real difference is that 160iu spills over in to the systemic circulation whereas the 40iu doesn't. This is unimportant for acute studies as the effects of peripheral and central insulin are identical before the development of CNS insulin resistance occurs.
Both allow facilitated ingress of calories in to peripheral cells, and help keep those calories there, until the cell signals that it wants no more.
With supplementary CNS insulin peripheral cells become "full" quicker, generate ROS sooner and resist insulin sooner. The brain senses that calories are no longer being taken up. So men stop eating sooner. This is not a central effect on appetite. It's a peripheral effect of allowing calories to fill peripheral cells sooner. So eating stops about (in the 160iu study) 200kcal prematurely. Adipocytes (and other cells) are full. The main difference compared with placebo is that there is less food present within the gut.
We know, at 40iu of intranasal insulin four times daily, that there is no weight loss over three weeks. After one dose of 160iu caloric intake after the first meal is reduced compared to placebo. If that were to translate to 600kcal/d (assuming three meals) there should be some weight loss. Ignoring changes to uncoupling, ie metabolic rate, this suggests that at subsequent meals there is a greater calorie intake, or more snacking between meals.
You could simply say that insulin makes you hungry (have I mentioned this before?). But that would be wrong. Extra insulin allows cell fullness to signal to the brain to stop eating when there is less food in the gut than there should be. Less food in the gut means you get hungry sooner. That means you eat more subsequently. By just enough to make up for the deficit. Because you skimped on a meal. So there is no weight loss for three weeks...
My hypothesis is that after three weeks on 40iu of intranasal insulin four times a day the VMH develops CNS insulin resistance. At this point the effect of intranasal insulin is lost. Also lost is the physiological CNS effect of augmented calorie storage derived from endogenous pancreatic insulin entering the brain.
People will then eat more at each meal because it takes longer for their peripheral cells to get full, so there will be more food in the gut at the time of satiation kicking in. But, with less CNS augmentation of insulin's peripheral action, lipolysis is free to proceed at a higher rate per unit insulin in the blood stream. So hunger is deferred by the augmented availability of stored FFAs. Oxidising these stored FFAs is, by definition, fat loss.
Men lose fat mass once they lose the CNS mediated fat storage effect of insulin.
That makes sense to me and explains why men who are already insulin resistant derive no benefit from intranasal insulin.
Peter
BTW, I'd expect the 40iu dose to work better long term than the 160iu dose provided the systemic leakage of insulin after 160iu outweighs the localised effect of developing insulin resistance within the CNS only. At four times the dose the CNS insulin resistance should occur sooner, to facilitate fat loss, but the systemic spill-over will always augment fat storage. Which would win?
The Hallschmid lab does not seem to have done a long term study of insulin at 160iu four times daily. It would be interesting to know what might have happened. Or maybe they did a pilot study and decided not to go there.
Edit. Oops, forgot the links!
Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man
Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin
End edit.
I've not found an acute study using 40iu but we can be pretty sure it works in the same manner as the acute 160iu dose. The only real difference is that 160iu spills over in to the systemic circulation whereas the 40iu doesn't. This is unimportant for acute studies as the effects of peripheral and central insulin are identical before the development of CNS insulin resistance occurs.
Both allow facilitated ingress of calories in to peripheral cells, and help keep those calories there, until the cell signals that it wants no more.
With supplementary CNS insulin peripheral cells become "full" quicker, generate ROS sooner and resist insulin sooner. The brain senses that calories are no longer being taken up. So men stop eating sooner. This is not a central effect on appetite. It's a peripheral effect of allowing calories to fill peripheral cells sooner. So eating stops about (in the 160iu study) 200kcal prematurely. Adipocytes (and other cells) are full. The main difference compared with placebo is that there is less food present within the gut.
We know, at 40iu of intranasal insulin four times daily, that there is no weight loss over three weeks. After one dose of 160iu caloric intake after the first meal is reduced compared to placebo. If that were to translate to 600kcal/d (assuming three meals) there should be some weight loss. Ignoring changes to uncoupling, ie metabolic rate, this suggests that at subsequent meals there is a greater calorie intake, or more snacking between meals.
You could simply say that insulin makes you hungry (have I mentioned this before?). But that would be wrong. Extra insulin allows cell fullness to signal to the brain to stop eating when there is less food in the gut than there should be. Less food in the gut means you get hungry sooner. That means you eat more subsequently. By just enough to make up for the deficit. Because you skimped on a meal. So there is no weight loss for three weeks...
My hypothesis is that after three weeks on 40iu of intranasal insulin four times a day the VMH develops CNS insulin resistance. At this point the effect of intranasal insulin is lost. Also lost is the physiological CNS effect of augmented calorie storage derived from endogenous pancreatic insulin entering the brain.
People will then eat more at each meal because it takes longer for their peripheral cells to get full, so there will be more food in the gut at the time of satiation kicking in. But, with less CNS augmentation of insulin's peripheral action, lipolysis is free to proceed at a higher rate per unit insulin in the blood stream. So hunger is deferred by the augmented availability of stored FFAs. Oxidising these stored FFAs is, by definition, fat loss.
Men lose fat mass once they lose the CNS mediated fat storage effect of insulin.
That makes sense to me and explains why men who are already insulin resistant derive no benefit from intranasal insulin.
Peter
BTW, I'd expect the 40iu dose to work better long term than the 160iu dose provided the systemic leakage of insulin after 160iu outweighs the localised effect of developing insulin resistance within the CNS only. At four times the dose the CNS insulin resistance should occur sooner, to facilitate fat loss, but the systemic spill-over will always augment fat storage. Which would win?
The Hallschmid lab does not seem to have done a long term study of insulin at 160iu four times daily. It would be interesting to know what might have happened. Or maybe they did a pilot study and decided not to go there.
Sunday, September 09, 2018
Insulin makes you hungry (7) superoxide is satiety
What causes satiety, if it's not insulin? This has to be understood if there is to be any chance of understanding the effects of intranasal insulin from the metabolic point of view.
Let's begin with individual cells, these are the entities which need to control metabolic substrate availability.
You eat some food. Plasma glucose and chylomicrons/FFAs rise, delivering energy to peripheral tissues. In the early stages of food absorption both glucose and FFAs enter cells under the facilitation of insulin. They do this easily, the cells are "hungry".
As an individual cell becomes replete it has to signal that it doesn't want any more metabolic substrate. This is achieved via the CoQ couple acting as the master sensor for metabolic energy status. It is being reduced using NADH from the cytoplasm (the glycerol-3-phosphate shuttle), by FADH2 input via complex II (acetyl-CoA in the TCA) and via FADH2 input from ETFdh (from beta oxidation of saturated fats). And of course from mitochondrial NADH via complex I. Given a high delta psi (ie minimal consumption of the proton motive force because ATP is already plentiful) this CoQ reduction eventually facilitates RET through complex I to give superoxide generation in order to stop insulin signalling. Which then limits cellular caloric ingress. This can be thought of as the "cellular satiety" signal. It is ROS generated. Let's say that again:
Satiety in peripheral cells is an ROS signal. It is generated in the mitochondria. This is pure Protons.
Now let's scale that up.
As more and more peripheral cells decide that they no longer need to respond to insulin then there is less and less of a "sump" available for absorbed calories to drop in to. The availability of calories which no longer have anywhere to go is the whole-body driver of the need to signal satiety. This surfeit of calories will be sensed in the VMH and the cessation of eating will be ROS mediated.
These people have the correct sort of idea:
Fuel utilization by hypothalamic neurons: roles for ROS
and so do these
Role for mitochondrial reactive oxygen species in brain lipid sensing: redox regulation of food intake
There is no need, to my mind, for CNS insulin to be involved. While insulin clearly has many effects in the brain, neurons do not appear to use insulin signalling to control caloric ingress, so insulin signalling should not influence ROS generation. Astrocytes might use insulin as peripheral cells do, but not neurons. After a meal, during food absorption, the brain can just get on with its normal insulin-induced physiological function, which is the facilitation of calorie storage in adipose (and other) tissues. Until they are full.
I'll make that clear: Satiety occurs when the brain senses that calories are no longer being accepted by the peripheral tissues using an ROS signal. Superoxide will be that signal.
That's what I consider to be the normal physiology.
We can now apply this to the various studies using intranasal insulin.
Peter
Let's begin with individual cells, these are the entities which need to control metabolic substrate availability.
You eat some food. Plasma glucose and chylomicrons/FFAs rise, delivering energy to peripheral tissues. In the early stages of food absorption both glucose and FFAs enter cells under the facilitation of insulin. They do this easily, the cells are "hungry".
As an individual cell becomes replete it has to signal that it doesn't want any more metabolic substrate. This is achieved via the CoQ couple acting as the master sensor for metabolic energy status. It is being reduced using NADH from the cytoplasm (the glycerol-3-phosphate shuttle), by FADH2 input via complex II (acetyl-CoA in the TCA) and via FADH2 input from ETFdh (from beta oxidation of saturated fats). And of course from mitochondrial NADH via complex I. Given a high delta psi (ie minimal consumption of the proton motive force because ATP is already plentiful) this CoQ reduction eventually facilitates RET through complex I to give superoxide generation in order to stop insulin signalling. Which then limits cellular caloric ingress. This can be thought of as the "cellular satiety" signal. It is ROS generated. Let's say that again:
Satiety in peripheral cells is an ROS signal. It is generated in the mitochondria. This is pure Protons.
Now let's scale that up.
As more and more peripheral cells decide that they no longer need to respond to insulin then there is less and less of a "sump" available for absorbed calories to drop in to. The availability of calories which no longer have anywhere to go is the whole-body driver of the need to signal satiety. This surfeit of calories will be sensed in the VMH and the cessation of eating will be ROS mediated.
These people have the correct sort of idea:
Fuel utilization by hypothalamic neurons: roles for ROS
and so do these
Role for mitochondrial reactive oxygen species in brain lipid sensing: redox regulation of food intake
I'll make that clear: Satiety occurs when the brain senses that calories are no longer being accepted by the peripheral tissues using an ROS signal. Superoxide will be that signal.
That's what I consider to be the normal physiology.
We can now apply this to the various studies using intranasal insulin.
Peter
Thursday, August 23, 2018
Insulin makes you hungry (6) except when you snort it
TLDR: The action of insulin is the inhibition of lipolysis.
This is our next paper:
Sniffing neuropeptides: a transnasal approach to the human brain
This is from 2002. Intranasal insulin does interesting things. I just wanted to run through the initial results following a single dose of 40iu of intranasal insulin and a subsequent (successful!) weight loss study based on this dose. These are the levels of insulin in cerebrospinal fluid (CSF) of volunteers as measured by sampling through a spinal catheter placed at the L4-L5 level (ouch!) and also in plasma after that single 40iu intranasal dose. It strikes me that the the insulin concentration in the rostral CNS might be a bit higher than down at L4 but I guess no one would volunteer for cisternal punctures/catheter placement to check this:
CSF insulin hits 25pmol/l. There is no penetration in to the systemic circulation. My feeling is that a rise from a basal CSF insulin of 7.0pmol/l to a peak of 25pmol/l, ie just over a tripling of fasting levels, probably reflects what might well be a physiological post-prandial concentration change within the brain. Roughly.
Note that intranasal insulin enters the brain at a clearly detectable concentration within ten minutes.
Then we have this study, also based around that 40iu intra-nasal dose:
Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man
A set of subjects, all male (perhaps the word "man" in the title should read "men". I'll give you one guess as to whether this trick for weight loss works in women. Or in insulin resistant "man/men" either), took 40iu of intranasal insulin, four times daily, for eight weeks. This will have been on top of their normal pancreatic responses to food over this period. Or they took placebo. This is what happened:
What was the effect of the first dose of intra-nasal insulin? Or, rather, from the first 28 doses? Not a lot, judging by the weight change in the first week. Or the second week. Or the third week. That's 84 doses of intranasal insulin over three weeks. Recall 40iu of intranasal insulin triples CSF insulin concentration within ten minutes. No weight loss over three weeks. Does anyone else see a trend for weight gain in the treatment arm (filled circles) over the first three weeks or is that just my confirmation bias over riding my visual acuity?
Recall that intranasal insulin enters the brain within ten minutes...
Anyway. For three weeks, that's 84 doses of 40iu intranasal insulin, there is zero weight loss. Nil. Zilch. Nadder. If anything there is a minuscule trend upwards. And I mean minuscule.
Then by week four something marvellous happens and consistent weight loss ensues.
So the question is: How many 40iu intranasal doses of insulin does it take to induced VMH insulin resistance in young healthy men? Sadly the answer is not 42, this is merely the answer to Life, the Universe and Everything. It's actually twice that, 84.
It seems that it takes more like 84 CNS over-exposures to insulin to obtund its lipolysis inhibiting effect normally induced via the VMH. At this point nutrients are less effectively sequestered in to adipocytes and so become more available for metabolism, obtunding hunger and allowing weight loss.
If anyone has a better explanation for the shape of the weight loss graph I'd be interested to hear it. The authors don't.
Peter
This is our next paper:
Sniffing neuropeptides: a transnasal approach to the human brain
This is from 2002. Intranasal insulin does interesting things. I just wanted to run through the initial results following a single dose of 40iu of intranasal insulin and a subsequent (successful!) weight loss study based on this dose. These are the levels of insulin in cerebrospinal fluid (CSF) of volunteers as measured by sampling through a spinal catheter placed at the L4-L5 level (ouch!) and also in plasma after that single 40iu intranasal dose. It strikes me that the the insulin concentration in the rostral CNS might be a bit higher than down at L4 but I guess no one would volunteer for cisternal punctures/catheter placement to check this:
CSF insulin hits 25pmol/l. There is no penetration in to the systemic circulation. My feeling is that a rise from a basal CSF insulin of 7.0pmol/l to a peak of 25pmol/l, ie just over a tripling of fasting levels, probably reflects what might well be a physiological post-prandial concentration change within the brain. Roughly.
Note that intranasal insulin enters the brain at a clearly detectable concentration within ten minutes.
Then we have this study, also based around that 40iu intra-nasal dose:
Manipulating central nervous mechanisms of food intake and body weight regulation by intranasal administration of neuropeptides in man
A set of subjects, all male (perhaps the word "man" in the title should read "men". I'll give you one guess as to whether this trick for weight loss works in women. Or in insulin resistant "man/men" either), took 40iu of intranasal insulin, four times daily, for eight weeks. This will have been on top of their normal pancreatic responses to food over this period. Or they took placebo. This is what happened:
What was the effect of the first dose of intra-nasal insulin? Or, rather, from the first 28 doses? Not a lot, judging by the weight change in the first week. Or the second week. Or the third week. That's 84 doses of intranasal insulin over three weeks. Recall 40iu of intranasal insulin triples CSF insulin concentration within ten minutes. No weight loss over three weeks. Does anyone else see a trend for weight gain in the treatment arm (filled circles) over the first three weeks or is that just my confirmation bias over riding my visual acuity?
Recall that intranasal insulin enters the brain within ten minutes...
Anyway. For three weeks, that's 84 doses of 40iu intranasal insulin, there is zero weight loss. Nil. Zilch. Nadder. If anything there is a minuscule trend upwards. And I mean minuscule.
Then by week four something marvellous happens and consistent weight loss ensues.
So the question is: How many 40iu intranasal doses of insulin does it take to induced VMH insulin resistance in young healthy men? Sadly the answer is not 42, this is merely the answer to Life, the Universe and Everything. It's actually twice that, 84.
It seems that it takes more like 84 CNS over-exposures to insulin to obtund its lipolysis inhibiting effect normally induced via the VMH. At this point nutrients are less effectively sequestered in to adipocytes and so become more available for metabolism, obtunding hunger and allowing weight loss.
If anyone has a better explanation for the shape of the weight loss graph I'd be interested to hear it. The authors don't.
Peter
Tuesday, August 21, 2018
Another brief housekeeping post
OK. I published the "awaiting moderation" comments. Lots of them are insightful and need a response. Time for this is not looking good at the moment! The main upset is that I mis-clicked delete instead of publish on a comment by Nicolás Flamel which I can't find any way of recovering. Sorry for that. Please feel free to re comment if you wish.
Peter
Peter
Insulin makes you hungry (5) except when you resist
TLDR: The function of insulin is the inhibition of lipolysis. Does resisting insulin facilitate lipolysis?
This the next paper:
Improving Influence of Insulin on Cognitive Functions in Humans
Aside: Oooooh, look. No control group! How can I already tell they are going to find that insulin suppresses appetite? End aside, sniggering excepted.
The only parts I am interested in are the clamps and the appetite scores (no food intakes in this one).
So we have a low insulin clamp and a high insulin clamp looking a lot like this:
These are the hunger ratings during the clamps:
I think we can say that hunger kicked in at around 150 minutes under the low dose insulin clamp and at around 300 minutes in the high dose insulin clamp. When would hunger kick in w/o insulin? We'll never know because...
So anyway, there we have it. Insulin, entering the brain by the physiological route, suppresses appetite in real live humans with a dose response. Time to shut up shop and go back to kayaking in my free time.
But just one moment. I think it might be worth looking at this study in the units of insulin concentration that we are most used to working with. I used this website to do the conversions.
We have here a fasting insulin somewhere around 40-50pmol/l. In old money that is 6.0microU/ml, quite reasonable.
The low clamp is around 900pmol/l, just over 130microU/ml. This is pretty well "normal" for physiological post prandial insulin after eating a meal of junk.
The "high" insulin clamp is around 30,000pmol/l (it's a log scale) by the six hour mark. I'll try to be careful with decimal points here, but I think this equates to just over 4000microU/ml. I'm not sure that even an insulinoma patient would run an insulin of 4000microU/ml. If anyone thinks this sort of insulin level has anything to do with appetite control in the physiology of humans, then they may be mistaken. But this level of insulin exposure does delay the onset of hunger, by over two hours in this study...
Now, here is a sideways way of looking at CNS insulin.
If the physiological role of insulin in the VMH is to augment fat storage, what might be the effect of CNS insulin resistance? Go on. I dare you to say that the effect might be partial failure to suppress lipolysis, less suppression of FFAs and so reduced appetite augmentation. Resisting insulin allows you to resist its hunger generating effects. How's that for a bizarre idea? Feel free to point out faults in the logic.
I've long been interested in the concept that exposure to insulin itself induces insulin resistance. There are a whole slew of papers to suggest this, some better than others. Overall I find the concept quite convincing.
If we want to actually see insulin resistance kick in rapidly, and measure it, we have to go to cell culture. Here we can overdose by decent amounts, using nanomoles rather than picomoles, and watch the reduction in signalling triggered by these high insulin concentrations. Insulin receptor (IR) phosphorylation drops within 45 minutes from the initial peak at 10 minutes after exposure. It's in this paper if anyone is interested in the details
Insulin Resistance Induced by Hyperinsulinemia Coincides with a Persistent Alteration at the Insulin Receptor Tyrosine Kinase Domain
but this is a typical graph:
If we convert the nanomolar concnetration from cell culture to the picomoles of in-vivo clamps then the two higher concentrations used in this graph would be 17,000pmol and 170,000pmol, similar to those from the high dose clamp used in the human study. Especially when you consider that 17,000pmol and 170,000pmol have indistinguishable effects on IR phosphorylation (and acute generation of resistance to that phosphorylation effect) in cell culture.
So it seems quite feasible to me that a physiological clamp at 900pmol/l would produce normal "insulin hunger" due to lipolysis suppression and that a clamp at 30,000pmol/l would induce significant insulin resistance. That limitation on insulin signalling would obtund the normal inhibition of lipolysis (but not eliminate it entirely) and so defer the normal onset of hunger routinely induced by the physiological action of insulin. So very large doses of insulin (170,000pmol in cell culture imitating 30,000pmol/l as a clamp) should postpone the onset of hunger compared to mildly supra-physiological doses (5000pmol in culture imitating 900pmol/l as a clamp) because the higher doses should cause less inhibition of lipolysis, with the proviso that you choose your target concentration very carefully. Obviously no one does a study without a pilot trial to make sure they will get the results they want. A saline infusion produces no significant increase in hunger over 150 minutes, though the trend in hunger is upwards because supper was a long time ago by the end of that particular study!
I'll take a pause here because I think this current concept is quite controversial enough to be stated as simply as possible. Obviously there are more studies which we can look at to explore the usefulness of this idea.
Peter
This the next paper:
Improving Influence of Insulin on Cognitive Functions in Humans
Aside: Oooooh, look. No control group! How can I already tell they are going to find that insulin suppresses appetite? End aside, sniggering excepted.
The only parts I am interested in are the clamps and the appetite scores (no food intakes in this one).
So we have a low insulin clamp and a high insulin clamp looking a lot like this:
These are the hunger ratings during the clamps:
I think we can say that hunger kicked in at around 150 minutes under the low dose insulin clamp and at around 300 minutes in the high dose insulin clamp. When would hunger kick in w/o insulin? We'll never know because...
So anyway, there we have it. Insulin, entering the brain by the physiological route, suppresses appetite in real live humans with a dose response. Time to shut up shop and go back to kayaking in my free time.
But just one moment. I think it might be worth looking at this study in the units of insulin concentration that we are most used to working with. I used this website to do the conversions.
We have here a fasting insulin somewhere around 40-50pmol/l. In old money that is 6.0microU/ml, quite reasonable.
The low clamp is around 900pmol/l, just over 130microU/ml. This is pretty well "normal" for physiological post prandial insulin after eating a meal of junk.
The "high" insulin clamp is around 30,000pmol/l (it's a log scale) by the six hour mark. I'll try to be careful with decimal points here, but I think this equates to just over 4000microU/ml. I'm not sure that even an insulinoma patient would run an insulin of 4000microU/ml. If anyone thinks this sort of insulin level has anything to do with appetite control in the physiology of humans, then they may be mistaken. But this level of insulin exposure does delay the onset of hunger, by over two hours in this study...
Now, here is a sideways way of looking at CNS insulin.
If the physiological role of insulin in the VMH is to augment fat storage, what might be the effect of CNS insulin resistance? Go on. I dare you to say that the effect might be partial failure to suppress lipolysis, less suppression of FFAs and so reduced appetite augmentation. Resisting insulin allows you to resist its hunger generating effects. How's that for a bizarre idea? Feel free to point out faults in the logic.
I've long been interested in the concept that exposure to insulin itself induces insulin resistance. There are a whole slew of papers to suggest this, some better than others. Overall I find the concept quite convincing.
If we want to actually see insulin resistance kick in rapidly, and measure it, we have to go to cell culture. Here we can overdose by decent amounts, using nanomoles rather than picomoles, and watch the reduction in signalling triggered by these high insulin concentrations. Insulin receptor (IR) phosphorylation drops within 45 minutes from the initial peak at 10 minutes after exposure. It's in this paper if anyone is interested in the details
Insulin Resistance Induced by Hyperinsulinemia Coincides with a Persistent Alteration at the Insulin Receptor Tyrosine Kinase Domain
but this is a typical graph:
If we convert the nanomolar concnetration from cell culture to the picomoles of in-vivo clamps then the two higher concentrations used in this graph would be 17,000pmol and 170,000pmol, similar to those from the high dose clamp used in the human study. Especially when you consider that 17,000pmol and 170,000pmol have indistinguishable effects on IR phosphorylation (and acute generation of resistance to that phosphorylation effect) in cell culture.
So it seems quite feasible to me that a physiological clamp at 900pmol/l would produce normal "insulin hunger" due to lipolysis suppression and that a clamp at 30,000pmol/l would induce significant insulin resistance. That limitation on insulin signalling would obtund the normal inhibition of lipolysis (but not eliminate it entirely) and so defer the normal onset of hunger routinely induced by the physiological action of insulin. So very large doses of insulin (170,000pmol in cell culture imitating 30,000pmol/l as a clamp) should postpone the onset of hunger compared to mildly supra-physiological doses (5000pmol in culture imitating 900pmol/l as a clamp) because the higher doses should cause less inhibition of lipolysis, with the proviso that you choose your target concentration very carefully. Obviously no one does a study without a pilot trial to make sure they will get the results they want. A saline infusion produces no significant increase in hunger over 150 minutes, though the trend in hunger is upwards because supper was a long time ago by the end of that particular study!
I'll take a pause here because I think this current concept is quite controversial enough to be stated as simply as possible. Obviously there are more studies which we can look at to explore the usefulness of this idea.
Peter
Friday, August 03, 2018
Holiday reading
I'm off on vacation for a while so the next posts are likely to be delayed. If anyone would like a light summary of the ideas I'm thinking about, this is a nice review:
Yin and Yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism
For a level deeper of understanding you just need to add in that saturated fats have an FADH2:NADH ratio around 0.49 which is the physiological signal for insulin resistance and MUFA generate one of around 0.47, giving the signal for insulin sensitivity. This is physiology.
Adding in PUFA as a bulk calorie source, with an insulin hyper-sensitivity generating FADH2:NADH ratio of well below 0.47, leads to expanded adipocytes. This is pathology.
Because a core function of insulin is the inhibition of lipolysis.
Peter
Yin and Yang of hypothalamic insulin and leptin signaling in regulating white adipose tissue metabolism
For a level deeper of understanding you just need to add in that saturated fats have an FADH2:NADH ratio around 0.49 which is the physiological signal for insulin resistance and MUFA generate one of around 0.47, giving the signal for insulin sensitivity. This is physiology.
Adding in PUFA as a bulk calorie source, with an insulin hyper-sensitivity generating FADH2:NADH ratio of well below 0.47, leads to expanded adipocytes. This is pathology.
Because a core function of insulin is the inhibition of lipolysis.
Peter
Wednesday, August 01, 2018
Insulin makes you hungry (4) unless you keep it out of your brain
TLDR: The function of insulin is the inhibition of lipolysis. Especially via the brain. Where insulin detemir doesn't go.
People will be aware that insulin detemir is really strange stuff. There are perfectly respectable papers showing that it cannot enter the brain (and blocks the entry of normal insulin in to the brain too) or that it is fantastic at entering the brain, much better than more normal insulins.
There are probably more studies in the latter camp but my biases push me towards the former camp. The nature of the researchers also tends to push me towards the former camp. I posted on insulin detemir here and here to explain my point of view.
Now there is this paper:
Euglycemic Infusion of Insulin Detemir Compared With Human Insulin Appears to Increase Direct Current Brain Potential Response and Reduces Food Intake While Inducing Similar Systemic Effects
OK. After an overnight fast, a 90 minute euglycaemic hyperinsulinaemic clamp and a 20 minute wait, subjects consistently eat less food (303kcal, 17% reduction) after an insulin detemir clamp (1475kcal) than they do after a neutral insulin clamp (1782kcal). Just eyeballing the insulin doses used we can assume that the plasma insulin levels were a reasonable approximation for humans in the normal post prandial period, ie physiological fed-state rather than pharmacological.
The research group is completely wedded to the idea that central insulin is an appetite suppressant and that weight gain from any insulin therapy is only a reaction to recurrent hypoglycamia. As there is no hypoglycaemia during the clamps their presumption is that this neutral insulin infusion results in a reduced food intake. As insulin detemir gives less food intake after a normoglycaemic clamp than neutral insulin does, then their conclusion is that insulin detemir is having a more potent central appetite suppressing effect than the neutral insulin.
They are so confident about this that the inclusion of a control situation, where saline was infused without any insulin and appetite was checked after this, was considered un-necessary. This really is the level of research in the "satiety" insulin camp.
Fortunately for us we do still have results from 1985 where food intake after a physiologoical post-prandial level clamp at 150microU/ml for 150 minutes using neutral insulin was compared to saline control and these give us this table cited in a previous post ("liquid drink" is a calorie containing soup-like food):
which allows us to calculate that saline reduces food intake by 40% compared to neutral insulin. Or to rephrase that more plausibly: a clamp using neutral insulin increases food intake by 60%. You can see why a control group was omitted by the "satiety from insulin" paper. I rather like insulin determir compared to any other insulin but you can see it has its work cut out to beat saline as a satiety hormone!
Simplest explanation: Insulin in the brain decreases peripheral lipolysis. This makes you hungry after an hyperinsulinaemic clamp. Insulin detemir doesn't enter the brain so has no CNS augmentation of its peripheral suppressive effect on lipolysis at a given level of glucose control, so it generates less hunger.
I'm a simple sort of a person. That's how I see it. Could be wrong of course.
Peter
People will be aware that insulin detemir is really strange stuff. There are perfectly respectable papers showing that it cannot enter the brain (and blocks the entry of normal insulin in to the brain too) or that it is fantastic at entering the brain, much better than more normal insulins.
There are probably more studies in the latter camp but my biases push me towards the former camp. The nature of the researchers also tends to push me towards the former camp. I posted on insulin detemir here and here to explain my point of view.
Now there is this paper:
Euglycemic Infusion of Insulin Detemir Compared With Human Insulin Appears to Increase Direct Current Brain Potential Response and Reduces Food Intake While Inducing Similar Systemic Effects
OK. After an overnight fast, a 90 minute euglycaemic hyperinsulinaemic clamp and a 20 minute wait, subjects consistently eat less food (303kcal, 17% reduction) after an insulin detemir clamp (1475kcal) than they do after a neutral insulin clamp (1782kcal). Just eyeballing the insulin doses used we can assume that the plasma insulin levels were a reasonable approximation for humans in the normal post prandial period, ie physiological fed-state rather than pharmacological.
The research group is completely wedded to the idea that central insulin is an appetite suppressant and that weight gain from any insulin therapy is only a reaction to recurrent hypoglycamia. As there is no hypoglycaemia during the clamps their presumption is that this neutral insulin infusion results in a reduced food intake. As insulin detemir gives less food intake after a normoglycaemic clamp than neutral insulin does, then their conclusion is that insulin detemir is having a more potent central appetite suppressing effect than the neutral insulin.
They are so confident about this that the inclusion of a control situation, where saline was infused without any insulin and appetite was checked after this, was considered un-necessary. This really is the level of research in the "satiety" insulin camp.
Fortunately for us we do still have results from 1985 where food intake after a physiologoical post-prandial level clamp at 150microU/ml for 150 minutes using neutral insulin was compared to saline control and these give us this table cited in a previous post ("liquid drink" is a calorie containing soup-like food):
which allows us to calculate that saline reduces food intake by 40% compared to neutral insulin. Or to rephrase that more plausibly: a clamp using neutral insulin increases food intake by 60%. You can see why a control group was omitted by the "satiety from insulin" paper. I rather like insulin determir compared to any other insulin but you can see it has its work cut out to beat saline as a satiety hormone!
Simplest explanation: Insulin in the brain decreases peripheral lipolysis. This makes you hungry after an hyperinsulinaemic clamp. Insulin detemir doesn't enter the brain so has no CNS augmentation of its peripheral suppressive effect on lipolysis at a given level of glucose control, so it generates less hunger.
I'm a simple sort of a person. That's how I see it. Could be wrong of course.
Peter
Monday, July 30, 2018
Insulin makes you hungry (3) a matter of semantics and free fatty acids
OK. There is absolutely nothing technically incorrect with the description of the results contained within the title of this paper:
Effects of insulin-induced hypoglycaemia on energy intake and food choice at a subsequent test meal
They gave a small dose of insulin (low enough to not need rescue glucose within the study period) as a single bolus, waited for 20 minutes then offered the subjects an eat-as-much-as you-like buffet. This is what the glucose levels did
and this is the subsequent food intake in kcal:
That will be 1700kcal after an hypoglycaemic glucose of 2.0mmol/l or 1400kcal after a blood glucose of 4.5mmol/l. Glucose drop and kcal increase are both p less than 0.05.
Hypoglycaemia makes you hungry.
Next is this one, not quite so good because the title omits the word "insulin", but never mind.
Short-term nocturnal hypoglycaemia increases morning food intake in healthy humans
Here they infused insulin to a glucose nadir of 2.2mmol/l, stopped the insulin infusion and then infused glucose to normalise blood glucose concentration within 30 minutes. On one occasion the nadir was induced at the start of REM sleep (as early as possible in the night) and on another occasion it was induced about 3.5 hours later. Total sleep time was about six and a half hours on each occasion. At feeding time all of the subjects were normoglycaemic. The white column is the control, the hatched is from when the hypo was about six hours before feeding and black is from when the hypo was about three hours before feeding.
The conclusion is that an hypo soon before you eat makes you hungry (p less than 0.05). An hypo just after you have fallen asleep the evening before might do as well but the p value is ns for this test. In general we can say any hypo leaves you hungry.
But both studies did two separate things. They generated hypoglycaemia and they gave insulin. The assumption is that it was the hypoglycaemia which drove the hunger. Even if the hypoglycaemia was long gone at feeding time.....
Hallschmid (second paper) is a dynamo of publications showing central insulin (via the intranasal route) is an appetite suppressor. You've noticed that this second paper did not have a group given insulin combined with intravenous glucose to protect against hypoglycaemia, isolating the effect of the insulin alone. But then we all know what that would have shown.
Let's look at this from the real world point of view, which is: The function of insulin is the inhibition of lipolysis.
What happened to FFA levels in either study? We'll never know.
Many years ago I posted on a Spanish study cited by Dr Davis. It has all of the pictures you might need embedded in the post. From this we can say that spiking your insulin by eating a small (40 gram) high carbohydrate snack will produce a rise in insulin from 50picomol/l (normal fasting) to around 70picomol/l (a little bit higher but not much, full post-prandial insulin would be at least several hundred picomol/l) at one hour, with return to 50picomol/l by two hours. Despite this tiny rise in insulin the FFAs drop from over 400micromol/l to 100micromol/l at two hours (by which time insulin has actually normalised) with persistence of some degree of hypolipidaemia for up to another three more hours. This is a tiny increment of insulin compared to the above studies.
We know that hypoglycaemia without hyperinsulinaemia does not drive appetite. My hypothesis is that it's the insulin itself which drives a fall in FFAs, which in turn drives the hunger to rise, all secondary to insulin's anti-lipolytic effect. Note, there is no need for any direct appetite modulation from insulin to explain these results.
So: you eat a Mars Bar at 15.30, just before evening consults ('cos you had a long theatre list, you had no lunch and there was nothing else to eat. Looong time ago, but I can still remember those days). By 17.30 you are ravenous and a bit shaky. Ha! Reactive hypoglycaemia! Oh, but your BG is 4.5mmol/l. Huh?
Best measure FFAs.............
Peter
Effects of insulin-induced hypoglycaemia on energy intake and food choice at a subsequent test meal
They gave a small dose of insulin (low enough to not need rescue glucose within the study period) as a single bolus, waited for 20 minutes then offered the subjects an eat-as-much-as you-like buffet. This is what the glucose levels did
and this is the subsequent food intake in kcal:
That will be 1700kcal after an hypoglycaemic glucose of 2.0mmol/l or 1400kcal after a blood glucose of 4.5mmol/l. Glucose drop and kcal increase are both p less than 0.05.
Hypoglycaemia makes you hungry.
Next is this one, not quite so good because the title omits the word "insulin", but never mind.
Short-term nocturnal hypoglycaemia increases morning food intake in healthy humans
Here they infused insulin to a glucose nadir of 2.2mmol/l, stopped the insulin infusion and then infused glucose to normalise blood glucose concentration within 30 minutes. On one occasion the nadir was induced at the start of REM sleep (as early as possible in the night) and on another occasion it was induced about 3.5 hours later. Total sleep time was about six and a half hours on each occasion. At feeding time all of the subjects were normoglycaemic. The white column is the control, the hatched is from when the hypo was about six hours before feeding and black is from when the hypo was about three hours before feeding.
The conclusion is that an hypo soon before you eat makes you hungry (p less than 0.05). An hypo just after you have fallen asleep the evening before might do as well but the p value is ns for this test. In general we can say any hypo leaves you hungry.
But both studies did two separate things. They generated hypoglycaemia and they gave insulin. The assumption is that it was the hypoglycaemia which drove the hunger. Even if the hypoglycaemia was long gone at feeding time.....
Hallschmid (second paper) is a dynamo of publications showing central insulin (via the intranasal route) is an appetite suppressor. You've noticed that this second paper did not have a group given insulin combined with intravenous glucose to protect against hypoglycaemia, isolating the effect of the insulin alone. But then we all know what that would have shown.
Let's look at this from the real world point of view, which is: The function of insulin is the inhibition of lipolysis.
What happened to FFA levels in either study? We'll never know.
Many years ago I posted on a Spanish study cited by Dr Davis. It has all of the pictures you might need embedded in the post. From this we can say that spiking your insulin by eating a small (40 gram) high carbohydrate snack will produce a rise in insulin from 50picomol/l (normal fasting) to around 70picomol/l (a little bit higher but not much, full post-prandial insulin would be at least several hundred picomol/l) at one hour, with return to 50picomol/l by two hours. Despite this tiny rise in insulin the FFAs drop from over 400micromol/l to 100micromol/l at two hours (by which time insulin has actually normalised) with persistence of some degree of hypolipidaemia for up to another three more hours. This is a tiny increment of insulin compared to the above studies.
We know that hypoglycaemia without hyperinsulinaemia does not drive appetite. My hypothesis is that it's the insulin itself which drives a fall in FFAs, which in turn drives the hunger to rise, all secondary to insulin's anti-lipolytic effect. Note, there is no need for any direct appetite modulation from insulin to explain these results.
So: you eat a Mars Bar at 15.30, just before evening consults ('cos you had a long theatre list, you had no lunch and there was nothing else to eat. Looong time ago, but I can still remember those days). By 17.30 you are ravenous and a bit shaky. Ha! Reactive hypoglycaemia! Oh, but your BG is 4.5mmol/l. Huh?
Best measure FFAs.............
Peter