Preamble. The best papers are those which challenge your ideas. When they conflict with what appears to be very hard evidence which support your mindset they become really exciting. Sometimes you just have to shrug, label the new finding as important and put it on the back burner to be ruminated about over the coming months. Sometimes a potential explanation is possible. This post is essentially a fairytale set in Denmark. It may be completely wrong. Or not. Here we go.
This paper came up in the comments to the last post from Gabor Erdosi via raphi. It is from Astrup's group in Denmark and I have to say I have a lot of time for Prof Astrup as he was one of the more influential people who objected to the gross stupidity of Denmark's transient fat tax a few years ago. The fat tax was abandoned quite rapidly as sensible EU dwelling Danes merely popped across their open border and stocked up with un-fat-taxed butter in Germany. Anyway:
The role of postprandial releases of insulin and incretin hormones in meal-induced satiety-effect of obesity and weight reduction
This is the crucial graph
Take 12 lean people, feed them a 600kcal sandwich for breakfast, wait just over three hours then offer them an ad-lib, well mixed pasta salad and see how much of this they eat.
The higher their insulin went after breakfast, the less they ate at lunchtime.
Insulin exposure is clearly associated with reduced subsequent food intake. You might be tempted to assume causality here, but you can't. It's an observational study of a very specific set of people. It can be used to generate an hypothesis, such as insulin suppresses subsequent food intake. But then you would have to test that hypothesis.
You could also simply go back through the literature to interventional studies which actually imposed changes in insulin levels and come to the opposite conclusion. Rodin et al did this here
Effect of insulin and glucose on feeding behavior
which makes that particular hypothesis untenable. Insulin makes you hungry.
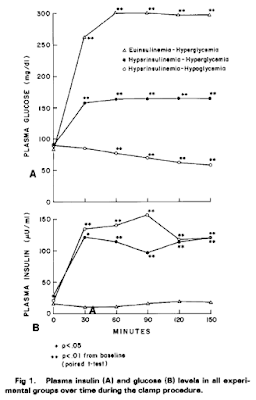
Here are the core findings, clamp values first
Here are the hunger ratings
and the amount of liquid food consumed, via a straw, through a screen:
To me personally, his study is very convincing. The principle is simple, logical and comprehensible. I would have been happier if he had also tracked FFAs in the study but we all know what insulin does to FFA levels (in the absence of fat ingestion). My personal view is that the brain looks at the availability of calories. Normoglycaemia with rapidly falling FFAs (the effect of insulin on adipocytes) is going to generate hunger. No one would expect any different. The action of insulin is the inhibition of lipolysis. Much higher levels are needed to facilitate the uptake of glucose.
We have a paradox, excellent. Direct insulin infusion makes you hungry. Insulin response to food makes you less hungry.
That is so cool.
Sooooo. Is it even remotely possible to explain the observation picked up by Prof Astrup in his 12 lean Danes? Starting from the basis that insulin drives calorie loss in to adipocytes with subsequent hunger? Speculation warning.
This group of Danes is very unusual. They have lived, on average, for 34 years in modern Denmark and they are not over weight. They have never counted a calorie, never been to Weight W@tchers, never had an eating disorder. They eat as much as they are comfortable with and eat again when they are hungry. If they pig out at Christmas they don't need to go on a diet in the New Year. They put zero effort in to being lean. That is a very special sort of person. They have normal appetite control.
When we give them a fixed calorie breakfast the insulin response varies. With these normal people I think it is a reasonable assumption that if the 600kcal is high compared to their preferred size of breakfast, the insulin level will go higher. There is more food than needed so more to store, that needs more insulin.
Members of the group who fancied many more than 600kcal for breakfast will have produced a low insulin response to the 600kcal specified by the study.
Now, it gets interesting because you cannot remotely account for a 1200kcal (3000kJ) difference in lunchtime eating by speculating about preferred breakfast size. The effect is too big.
The storage of calories is the simplest of actions of insulin. It does other things too. For these we have to go to the very, very artificial model of Veech's isolated working rat hearts. However some of the findings do have some bearing on real life.
In this paper
Substrate signaling by insulin: a ketone bodies ratio mimics insulin action in heart
we have this snippet:
"Unexpectedly, insulin increased cardiac hydraulic work but decreased net glycolytic flux and O2 consumption, improving net cardiac efficiency by 28%".
Insulin facilitates glucose diffusion in to the myocytes but partitions it in to stored glycogen. Glycolysis actually decreases but there is an increase in efficiency which gives the 28% increase in cardiac work.
Let that sink in. Insulin makes energy production more efficient while diverting calories in to storage. If you wanted to fatten someone up that seems like a good plan.
From a related paper by Veech's group
Insulin, ketone bodies, and mitochondrial energy transduction
we have a slight elaboration:
"The increase in efficiency associated with insulin administration is not readily explained by such a straightforward mechanism [as for ketones]; other factors, such as reduction of the mitochondrial NAD couple or specific effects like covalent modification of mitochondrial membrane protein, will have to be considered as possible factors altering efficiency of ATP synthesis".
Veech's model runs on glucose alone but there will undoubtedly be residual FFAs in the cardiac myocytes. You just have to wonder whether the effect of insulin is to extract these from the mitochondrial uncoupling proteins and covalently bind them in to intracellular triglycerides. An interesting idea and it would certainly tighten the coupling of the ETC.
Bottom line: Insulin, when working as it should, diverts calories to storage but increases efficiency of energy production to allow normal metabolism. I hold that this happens in the elevated insulin individuals of the lean subject group. Recall that all of these people are naturally lean. When the insulin wears off they realise, metabolically, that they have gained a (very) little weight while running a very efficient metabolism. If they have extra stored calories which, being naturally lean, they don't need, why should they eat much at lunch time?
The hypoinsulinaemic lean people get their 600kcal, decide it is way too little to bother storing and partition it towards utilisation. There is no drive towards storage, very little insulin, no insulin mediated increased efficiency. Substrate is available, it gets oxidised. Very little gets stored. This is the low insulin state. When lunch is presented to this naturally weight stable person their metabolism realises that it has not maintained fat stores after the 600kcal breakfast so they eat more at lunch time.
We have a period of high efficiency calorie conservation in the high insulin group and a period of low efficiency calorie wastage in the low insulin individuals. Because these people have that rare gem, a functional metabolism, they simply adjust subsequent food intake to reflect their previous fuel partitioning during the three hours from breakfast to lunch.
It is perfectly reasonable to mistake this scenario as an indicator that insulin is a satiety signal. Easily done. It's incorrect.
Peter
The invariable after-thought: Does insulin correlate inversely with reduced food intake in either the obese group at the start of the study or after marked weight loss?
No. Of course not.



I would think from this paper that for people if their metabolism is not broken, insulin tends to increase satiety. These people remain lean, because for them the hormonal system is working properly. But for those people whose metabolism is broken (almost everybody these days), this does not work. And so they tend to eat more because their hunger signals are all out of whack. So it is probably better to eat something that gives better satiety. For many people it would be a low carb diet. But for people like Jimmy Moore, the low carb diet is not providing satiety, so they tend to get fat. I do think a high protein diet will essentially force satiety, because protein in excess is difficult for the body to use or store. This will work regardless of the person. But for all other diets individual response matters.
ReplyDeletere: …standardised, solid test-meal, consisting of a sandwich of 135 g wheat bread with…
ReplyDeleteWhen appetite stimulation is the topic, wheat needs to be off the table, because it's not just 60% rapidly-metabolized branched-chain glucose. The proteins are also known to independently provoke appetite.
The other insights into what might have been learned here aside, it would have been a less confounded trial if they'd used an iso-glycemic gluten-free bread, or just sugar.
Fig 1 in Rodin seems to have labels switched. A is glucose and B is insulin.
ReplyDeleteHi Gretchen,
ReplyDeleteI've had a look and I think probably not. It is a little confusing because she labels both 150mg/dl and 300mg/dl as "hyperglycaemia". I see triangles as low (ie normal) insulin high glucose (300mg/dl), solid circles as high insulin high glucose (150-ishmg/dl) and open circles as high insulin low (70-ish mg/dl) glucose. Control group not shown. That makes sense but I agree, you have to look at the lines very carefully!
Boundless, yes, although I would phrase it differently. More like wheat flour contains an insulin mimetic which drives calories in to storage, resulting in secondary hunger. Same statement but looking at it at a more basic biochemical level. Stops you having to worry about mechanism.
Anand, interesting. Do you see Rodin's results as invalid?
Peter
Hello Peter,
ReplyDeletewhat bothers me about the Danish study is the clear line they drew between lean and obese. (I do not think these men were lean but rather very well-nourished. That body fat?) Anyway, look at their BG and Insulin at baseline.
Not every lean person has such good BG. And being obese does not mean metabolically deranged. It seems to me they selected their participants to fit the stereotypical "lean" and "obese". The obese did a reduction diet. My guess is they did that because their insulin/BG were not right to begin with.
The results have nothing to do with leanness or obesity or weight reduction but with insulin response. The title is misleading. I think.
I believe stacking the deck is quite normal in this field...
ReplyDeletePeter
Could another explanation be that the people whose adipocytes are the most insulin resistant produced the most insulin to store away all of the glucose? Those same people would then have the highest FFAs, presumably, and would consume less at a the ad lib meal.
ReplyDeleteHi Brad, yes, you could make that case...
ReplyDeletePeter