People may have noticed that I'm quite interested in basal lipolysis, adipocyte size and metabolic syndrome. That is correct.
Hypophagia and metabolic adaptations in mice with defective ATGL-mediated lipolysis cause resistance to HFD-induced obesity
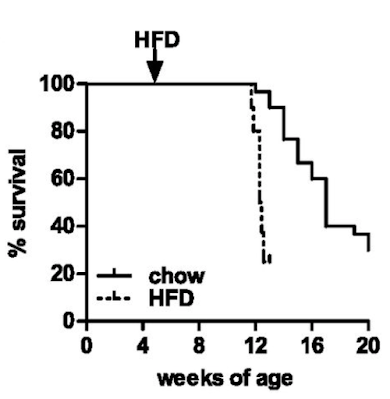
Well, they die. Not unexpected. Here are the survival curves, one group of ATGL knockouts fed chow and another fed an "high fat diet" based on a modified D12492 (mostly extra sucrose with the lard)
If you consider which organ runs (in health) almost exclusively on fatty acid oxidation it will come as no surprise that the mice die of dilated cardiomyopathy secondary to lipid accumulation and mitochondrial failure. Sooner and more rapidly on the high fat diet.
You can get round this problem by engineering the ATGL gene just in to cardiac myocytes. Then the animals live long enough to allow you to study the effects of ATGL deficiency in the absence of a dead myocardium. The whole of the paper, other than Figure S1, uses mice with this protected myocardium (denoted cTg). WT/cTg denotes normal ATGL throughout their body plus extra myocardial ATGL (phenotypically normal) or AKO/cTg without ATGL everywhere other than their myocardium. So ignore the cTg label part, its WT vs AKO re adipocytes throughout the paper
So here's the paradox.
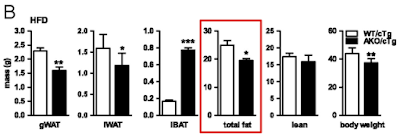
On chow WT mice carry approximately 2g of fat and AKO mice carry 5g of fat, much as the histology suggests and as you might expect. Figure 1 summarises the top left and top right groups of mice:
Things get more interesting when we compare the high fat fed WT mice with the high fat fed AKO mice, thats the bottom left and bottom right. Both groups have increased fat mass but the AKO mice are less obese than the WT mice. Like this
Note that all of the vertical scales are different. But there we have it, knocking out ATGL long term blunts the obesity induced by D12492, somewhat. The effect kicks in slowly but is well established by the end of the study at 22 weeks (solid black squares are AKO)
The paper goes in to some detail about PPAR-γ2 suppression in AKO mice which can be reverse by the diabetes PPAR agonist rosiglitazone.
Which ultimately translates as the adipocytes adapt to being unable to offload triglycerides by suppressing every aspect of lipid uptake and storage that they can.
"... the expression of genes involved in lipogenesis and fat storage such as PPAR-γ2 (−95%), C/EBPα (−30%), and SREBP1c (−78%) were significantly lower in gWAT from HFD-fed AKO/cTg mice than from WT/cTg."
The AKO mouse adipocytes, which cannot off-load lipid, compensate by progressively rejecting lipid ingress.
Does this make the adipocytes insulin resistant? Or the mice insulin resistant?
They didn't look at this at the adipocyte level and the interactions are too complex to guess how adipocytes might respond to physiological or pharmacological exposure to insulin.
What we do know is that the AKO mice fed a high fat diet are still very insulin sensitive at the whole body level despite their adipocytes eventually down regulating all aspects of lipid accumulation. Here's the intra-peritoneal glucose tolerance test result. All of the following results are high fat diet based.
If you can read Table S1 in the original paper (too faint to reproduce here) you can see that fasting insulin in the AKO mice is 0.1ng/ml vs WT at 1.0ng/ml. Fasting glucose is also low at 164mg/dl in the AKO mice vs 212mg/dl in WT. Sorry for all the Noddy units. HOMA-IR score would be very, very low for AKO mice.
It doesn't matter what size the adipocytes of an AKO mouse are, they are not going to perform basal lipolysis. Under fasting conditions in normal mice FFAs go up as augmented lipolysis frees FFAs and there is little insulin to limit further lipolysis and FFA release. We need elevated FFAs under fasting.
In Table S1 again the WT mice have a fed FFA level of 0.72mM which rises to 0.93mM on a 4h fast, as it should do. In the AKO mice fed FFAs are 0.65mM and drop to 0.45mM on a 4h fast. They drop on fasting, so we can assume that the initial 0.65mM fed value is largely diet derived and so, with no food and no basal lipolysis, FFA levels have to fall.
Which they do.
My premise from Protons is that insulin resistance is an adaptive response to the delivery of FFAs. Under fasting this is ideal. In the presence of elevated glucose and insulin then the elevated FFAs from distended adipocytes cause caloric oversupply to the whole body and insulin resistance has to kick in to adapt. It is an antioxidant defence mechanism to limit ROS generation to physiological levels.
No ATGL -> perennialy low FFAs -> no need to resist insulin -> insulin sensitive
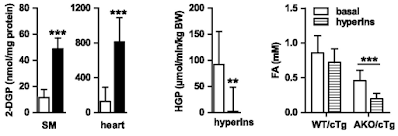
If we look at the hyperinsulinaemic clamp data we can see that both skeletal muscle (SM) and heart in AKO mice are really good at taking up 2-deoxyglucose. The liver, on a diet of 28% sucrose by weight, is also *very* insulin sensitive, with near total suppression of hepatic glucose production (HGP) during the clamp:
Of course the interesting bar chart is the right hand end one. The basal FFA levels are the ones cited above after a 4h fast. Hyperinsulinaemia with normoglycaemia lowers fasting FFAs a little, but without statistical significance, in WT mice obese from D12492. Doing the same in AKO mice produces a marked fall from low levels to even lower levels, probably somewhere around 0.2mM.
Clearly the excess of plasma free fatty acids, derived from elevated basal lipolysis and which necessitate insulin resistance ie trigger metabolic syndrome, is not present in the AKO mice. Whatever the size of their adipocyte lipid droplets there is no fatty acid release. My guess for the fall in FFAs is that residual post prandial FFAs are being allowed in to muscle and liver cells using CD36 translocated to the cell surface in parallel to GLUT4s in response to the clamp.
Asides before I finish: What does the term "hypophagia" in the title of the paper actually mean? It means that the mice are NOT HUNGRY. They eat ad lib until they are satiated. Because they have down regulated their ability to "sequester" calories in to adipocytes, they sense adequate calories are available earlier so stop eating earlier. They are not "hypophagic", their lack of hunger is manifest as eating less. They're not under-eating. They're eating exactly the correct amount of food to supply their metabolic needs. It doesn't matter that the food appears to be hedonistic, rewarding or addictive (stop sniggering) as it appears to be in the WT mice. Under exactly the same hedonistic/rewarding/addictive food environment (you really must stop sniggering, and so must I) as the WT mice the AKO mice are simply NOT HUNGRY. The brain is such a secondary organ compared to the adipocyte.
Another aside: Where does hepatic insulin resistance in fructose fed mice (like the WT here) come from? Look here
Yes. Fructose, if it gets as far as adipocytes, forces FFA release. This will use ATGL. These FFAs will end up in the liver and have to be repackaged as VLDLs to be returned to the adipocytes. If insulin sensitivity is pathologically high (ie linoleic acid exposure) those FFAs in the liver will be stored there giving fatty liver, NAFLD etc. The AKO mice will obviously catabolise fructose without any problem but will be incapable of fatty liver because they cannot transfer fatty acids out of adipocytes to get to hepatocytes. Hence the incredible ability to suppress hepatic glucose production during the clamp in AKO mice. See HGP in the above figure. Adipocyte AGTL is essential for NAFLD on an high fructose diet.
Yet another aside: Ethanol does exactly the same as fructose, same mechanism:
Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis
Chronic alcohol exposure stimulates adipose tissue lipolysis in mice: role of reverse triglyceride transport in the pathogenesis of alcoholic steatosis
Okay, I'll shut up now. The role of ATGL in converting linoleic acid induced insulin sensitisation in to whole body insulin resistance and metabolic syndrome is central. This extends to NAFLD and ALD.
Physiology is comprehensible.
Peter
I was going to hit post but two more asides have presented themselves to my brain. Rosiglitazone more than eliminates the down regulation of PPAR-γ2 in AKO mice to give a slightly more obese mouse than the WT high fat fed mice. Does this restore insulin resistance too? Of course not, those bigger adipocytes still can't do lipolysis. The group must know this so they simply didn't run IPGTTs on the rosi-fat mice.
Also re adipocytes under clamp conditions. They looked at behaviour of skeletal muscle cells, heart cells and liver cells under hyperinsulinaemia. But not adipocytes. They know these adipocytes are resistant to insulin, so they didn't check. In a paper on AGTL and adipocyte function. They know this. I love glaring holes from carefully crafted methods sections where you can see the not-investigated leverages.
Oh, and at the time of this study they were clearly looking to find a drug to recreated the benefits of AGTL knockout on weight gain (for high PUFA, high sucrose fed humans). If they had found one I guess they would just have crossed their fingers that it wouldn't trigger dilated cardiomyopathy.
Oh, and another. High PUFA, high sucrose diets clearly do not trigger insulin resistance in the absence of pathologically distended adipocytes forcing elevated basal lipolysis and caloric overload induced high delta psi. The high linoleic acid only generates the 4-HNE to augment insulin resistance and ultimately shut down ETC function when caloric supply and delta psi is excessive. These AKO mice have access to obesogenic levels of PUFA and 21% atmospheric oxygen, yet essentially zero insulin resistance.
Now I really will stop.




I don't want to derail your lipid discussions, but can you
ReplyDeletetake a minute or two to give your thoughts about Dr. Michael
Eades's video at Low Carb Down Under? It's about mass instead
of calories: https://www.youtube.com/watch?v=0mnhha9JfCM&t=6s
Thanks.
Peter, I think your theory that insulin resitance is necessary in fasting, can be right, but at what level of insulin? The point is that Malonyl-CoA as main inhibitor of fat entry to mitochondria is regulated by insulin. Elevated fasting insulin suppress a little bit intake of fat, so elevates FFA. This little suppression of fat burning allows more glucose to´be burned in fasting, more Malonyl-CoA, with low ROS and low insulin sensitivity. I dont think this is good. All FFA should be allowed to mitochondria in fasting, it should not be limited. When limited, peroxisomes take place instead of mitochondria and this triggers fattening. PUFAs go first to peroxisomes when insulin is little elevated, so they worsen the situation. My suggestion.
ReplyDeleteJaromir
You may find it interesting
"Insulin Inhibits Peroxisomal Fatty Acid Oxidation in Isolated Rat Hepatocytes*
https://academic.oup.com/endo/article/142/6/2702/2989741
So central to T2D is insulin tolerance (called insulin resistance - but tolerance is clearer - to much of something creates tolerance).
ReplyDeleteIn the end it becomes clear that refined sugar, artificial sweeteners, and seed oils are not human foods that we evolved to eat.
In the heart group I'm involved with, I talk about my 'great grandmother rule' a heuristic (rule of thumb) - don't eat anything your great grandmother didn't eat. (in her time - T2D was not at all common). This means no Boxed foods - no foods with additives etc - no CIAB..
The reason for the Heuristic is that it is close to impossible to make sense out of nutritional research - to many 'cooked' paper - poorly designed papers etc.
,.,.
I spent some time looking for papers that might explain why livestock gains weight faster on antibiotics - not clear that we know the mechanism. This seems important.
When I was young, they sold day-old-bread - it was already not as good - it spoiled. Today, the bread stays on the shelf for a week. The ingredient is not listed on the package (less than 500mg/serving). These are called preservatives - but what do preservatives do? They prevent growth of biotics - and could also be called antibiotics.
This reframing begs the question - Are we inducing weight gain with a type of antibiotic in our foods?
@bill
ReplyDeleteDespite what he says in the clips - I did not easily find the list of papers - had to find them the hard way.
https://www.cell.com/heliyon/fulltext/S2405-8440(20)31048-3
https://www.medrxiv.org/content/10.1101/2020.10.27.20220202v10
https://www.preprints.org/manuscript/202208.0309/v12
https://www.sciencedirect.com/science/article/pii/S0022519322002351
https://www.medrxiv.org/content/10.1101/2020.10.27.20220202v4
At the end, I found his links
https://www.proteinpower.com/lowcarbusaboca/
If I understand? They were looking at energy rather than mass? I thought the respiration work measured the CO2 - what am I missing?
the only people looking for and talking about paradoxes are Gary Taubes and yourself. Really says a lot about the state of nutrition research... I love those posts because after a few paragraphs I find myself muttering "Right, where's my blackboard?"
ReplyDeletebill, I think the idea applies under fixed caloric conditions and I've followed Mikes links to the two papers. As Mike says, the math is utterly beyond the average person. If it was simple I think it would be more self explanatory.
ReplyDeleteJohn Speakman:
"When people lose weight by ⬇️ carbs & ⬆️ protein without cutting calories they lied on the diet report sheets."
Understanding the math would possibly help here. Otherwise low carb-ing (but not low fat-ing) makes you a liar. Hmmmmmm...
Peter
Just an interesting snippet, that's the John Speakman of doublely-labelled water renown. He should know. But he also knows all about the metabolic effects of F3666 on mice and has a knack of excluding this effect while drawing an highly misleading straight line through a weight gain inverted "U" curve. No lying, just very?????
ReplyDeletehttps://high-fat-nutrition.blogspot.com/2019/11/of-mice-and-men.html
Adv extreme caution.
Peter
raphi, I hope it makes sense.
ReplyDeleteFor HFD (sucrose PUFA-lard) fed mice:
Of course limiting lipogenesis in AKO mice could easily be substituted in normal mice by reducing insulin signalling: ie metformin anybody?
Or, gasp, by not eating carbs.
Peter
If I think of T2D as tolerance to insulin caused by replacing food with 'food-like-substances' - or in yet another framing - drug like additives found in CIAB/ultra processed foods - I see things a bit differently than mainstream thought. The chronically elevated insulin causes all sorts of problems - obesity, sodium retention - perhaps acts as a growth factor with yet other bad outcomes (thickened intima that becomes hypoxic?)
ReplyDeleteSo we have consumption of what can be thought of as drugs(sugar, sweeteners, process food additives) that act as a monkey-wrench in the clock works - and so the answer is to take yet another drug?(metformin) instead of fixing the actual cause? I don't think so.
Most pharma drugs work as selective toxins - blocking some pathway. This can be life saving (antibiotics), but the promises of good with a drug for every symptom has been greatly oversold. The idea that medicine should be focused on treating with drugs seems like a big mistake.
I am taken by Eades's question - why are we looking at energy units for weight gain instead of mass? I am familiar with the 'bomb calorimeter' technique used to measure energy content in food - but I've always had a worry; burning up food is not quite the same as digestion. We don't poop cinders. What would matter is the energy left over from gut flora digestion, but while we harvest the energy in chemical bonds in food - weight gain is an increase in mass - not energy. (I don't understand why he drifted off to nuclear energy in his talk - for me, it stirred up misgivings about what he was saying).
So if different foods are digested differently, we have mass-in vs mass-out - measured as fluid-loss+poop-loss+exhaled-loss. Is it really true that no one has really looked at this for different food types? I know that people did 'bomb calorimeter' work with poop. There was also work measuring exhaled CO2 - confounded with water loss. Somehow I believed that this had been sorted out?
Reminds me of this:'Measuring human digestive efficiency vs. a flame'
https://www.youtube.com/watch?v=9wZ0wTqJIxY
Hi Jaromir,
ReplyDeleteFinally got chance to look at the paper and yes, it's interesting. It opens up all sorts of avenues.
Peter
karl, I started looking at the mass of oxygen taken up as "mass-in" as well as "mass-out" in CO2 and H20. But the mathematicians have worked all that in too. I just wish I could follow the math!
ReplyDeletePeter
Hi Peter,
ReplyDeleteand patological PUFA insulin sensitivity can be perfectly explained by peroxisomal PUFA beta oxidation, which elevates H2O2, let glocose into the ćell and blocks saturated fat beta oxidation in mitochondria by malonyl-CoA. Elevates acetate and acetyl-CoA, so this triggers fattening process. It looks very straitforward and simple now.
Jaromi
And I think nature intended this process for higher insulin levels, but PUFAs shift this fattening process to much lower insulin levels.
ReplyDeleteJaromir
Peter, whenever I read about mass in/mass out (which seems to be a sockpuppet for cico) I always wonder how fat a tiger would become when force fed a diet of chaff? The sort of thing which would fatten a horse up nicely.
ReplyDeleteI'm not the least bit confident that you can extrapolate from poor old mutant C57BL6 to humans however when I look at your inverted U curve in that linked post, if you ignore the very low confidence central data point and draw a fresh curve heading down through the next two points
up from your estimate, it looks to my eyes that the weight dips even lower at the high fat end.
The inverted U curve also supports my recent musing, that if you do increase the carb amount in your diet you would need to drop the fat calorie percent by more than that increase in order not to end up approaching the heavier central part of the curve. The same would be true in the opposite direction, ie from the high carb end, devil in the details of course.
Hmmmm, that last paragraph may need revision.
ReplyDeleteAnd problems only arise when glutathione runs out, complex I is acetylated, and increased lactate from glycolysis shuts down peroxisomes. Then there will be a lack of H2O2 and insulin resistance. Peroxisomes do not produce NADH but actually lactate. An excess of lactate stops them and triggers HIF-1 and pseudohypoxia, this completely changes the metabolism. Now that is almost complete.
ReplyDeleteJaromir
karl—"monkey-wrench in the clock works" is exactly how I view pharmaceuticals, which can be thought of as intentional low-dose poisoning.
ReplyDelete"This reframing begs the question - Are we inducing weight gain with a type of antibiotic [preservatives] in our foods?" What an intriguing thought. In the case of livestock and antibiotics, of course it must be causing some kind of dysbiosis, but I figure you're wanting more detail than that.
Off topic in some ways, not entirely, I was browing for info about PFAS's, this site has a good overview:
ReplyDeletehttps://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm
Looking at their sketch of the C8 PFAS for instance, it is exactly a lipid except that all the hydrogens on the backbone have been replaced by fluorine. This is a molecule from hell, sets me to thinking about how this could easily have multiple serious metabolic outcomes ie everywhere that a regular lipid is expected one of these bastards could turn up.
Hell.
Intermittent fasting? OMAD? You're doing it all wrong!
ReplyDelete'Night cereal: It’s hard that food corporations have only three meals a day to shovel corn and vegetable oil down our gullets. To solve for this, they have invented a new meal: bedtime cereal. “Post Consumer Brands is looking to help make your sleep dreams come true with Sweet Dreams—the first ready-to-eat cereal designed to be part of a healthy sleep routine,” the marketing copy reads. At 10 p.m., when you are watching YouTube, slack-jawed and looking like the peak of sleep hygiene, you might as well complete the scene with some Sweet Dreams Honey Moonglow.
'In what can only be described as a hate crime against millennial women, they call the night cereal “self-care.” From that same press release: “ ‘More than ever, consumers are looking to embrace acts of self-care, particularly as it relates to bedtime routines and we believe a relaxing bedtime routine is key to a good night’s sleep,’ said Logan Sohn, Senior Brand Manager.”'
From https://www.thefp.com/p/tgif-let-them-eat-night-cereal
Hi Jaromir,
ReplyDeleteJust followed up links on peroxisomes and lactate. Nice. I'd phrase it slightly differently in that peroxisomes export the hydrogen generated in peroxisomal oxidation attached to pyruvate as lactate. Obviously glycolysis to the point of lactate generation (typical of fructose) will alter the redox balance to deplete pyruvate and favour lactate. Interesting. There seem to be a few other similar shuttles too but pyruvate/lactate looks very significant, especially as it is possible to view lactate as the preferred mitochondrial fuel vs pyruvate...
Ta.
Peter