Visceral adipocytes are exquisitely insulin sensitive. Visceral adipocytes are insulin resistant. Let's go.
This is another of the anchor studies which has informed my world view over the last decade or so:
Insulin signaling in human visceral and subcutaneous adipose tissue in vivo
This is another of the anchor studies which has informed my world view over the last decade or so:
Insulin signaling in human visceral and subcutaneous adipose tissue in vivo
I like it because it used real live intact human beings, it used a physiological dose of insulin and it looked at the signalling pathways within cells. It then compared the effects within visceral adipocytes (omental in this case) with those in subcutaneous adipocytes. I'm not sure that I had much of an opinion about this before I read the study but it locked me in to my still current opinion that visceral adipocytes are particularly insulin sensitive.
This has been reinforced by the PET scan data from this next study. The mouse section is poor as it compares SC adipose tissue to epididymal adipose, the effect would be more marked if they had used omental/mesenteric as the visceral adipose source but hey, the PET data in real live humans are nice...
This has been reinforced by the PET scan data from this next study. The mouse section is poor as it compares SC adipose tissue to epididymal adipose, the effect would be more marked if they had used omental/mesenteric as the visceral adipose source but hey, the PET data in real live humans are nice...
Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging
This property of visceral adipocytes appears to be intrinsic to their stem cells. If you differentiate stem cells from SC and visceral tissues in to adipocytes those adipocytes, generated in-vitro, retain this exquisite insulin sensitivity.
Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells
This property of visceral adipocytes appears to be intrinsic to their stem cells. If you differentiate stem cells from SC and visceral tissues in to adipocytes those adipocytes, generated in-vitro, retain this exquisite insulin sensitivity.
Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells
So visceral adipocytes are the most insulin sensitive in the body and this is programmed in to their stem cells, which makes me accept that it is evolutionarily conserved and so essential their function. Which, surprisingly, is neither to kill us nor give us metabolic syndrome.
But we also have this:
All of the below quotes are taken from the same screenshot.
This was 2010. I doubt that there is any less confusion nowadays.
"Adipocytes from VAT are more insulin-resistant than SCAT adipocytes (39,40)."
"Visceral adipose tissue has higher rate of insulin-stimulated glucose uptake compared with SCAT adipocytes."
"Visceral adipocytes are more metabolically active and have a greater lipolytic activity than SCAT adipocytes (44,45)."
"Visceral adipose tissue has higher rate of insulin-stimulated glucose uptake compared with SCAT adipocytes."
"Visceral adipocytes are more metabolically active and have a greater lipolytic activity than SCAT adipocytes (44,45)."
For my sins I started with reference 44, Arner's 1995 paper
Differences in lipolysis between human subcutaneous and omental adipose tissues
Differences in lipolysis between human subcutaneous and omental adipose tissues
which had slightly weird methods for investigating adipose tissue function. This referred me to the same group's 1983 paper
Differences at the receptor and postreceptor levels between human omental and subcutaneous adipose tissue in the action of insulin on lipolysis
Differences at the receptor and postreceptor levels between human omental and subcutaneous adipose tissue in the action of insulin on lipolysis
and finally back to Arner 1981
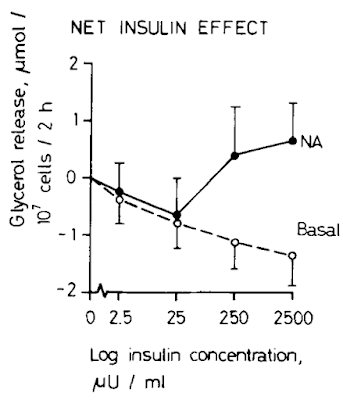
which gave me this image:
The lines represent paired adipose samples, a control sample labelled "Basal" (open circles) and a sample to which a fixed concentration of noradrenaline (NA) had been added at all insulin exposures.
The first red flag is that basal lipolysis can be suppressed by insulin. So what this paper means by basal lipolysis and what other papers mean by basal lipolysis appear to be quite different. The other really strange finding is that under noradrenaline induced lipolysis insulin is suppressive (as you would expect) up to 250μU/ml at which point insulin enhances lipolysis, markedly. Hmmmmmm. I would suggest insulin induced insulin resistance but that seems like a long shot. Ultimately the model doesn't seem to be a very good one.
The only difference I can see between this group's model and the rest of the world is that they use tiny cubes of adipose tissue rather than isolated adipocytes. If I had to guess I would suggest that there are the end terminals of noradrenergic neurons present in their adipose samples and these are modifying the response to insulin and noradrenalin. It could also be that having structure around adipocytes might alter their behaviour ie this might be a better model than isolated adipocytes. Hard to compare when almost all of the surrounding work is done with isolated adipocytes. Generally I would be very cautious about looking for understanding in such a model as the results are counterintuitive, but I could be wrong. That's a few days of my free time I'll never get back! Next I checked the other three refs.
Ref 45
Metabolic complications of visceral obesity: contribution to the etiology of life of type 2 diabetes and implications for prevention and treatment.
is a review and not available on Pubmed or Sci-Hub and I doubt it it's worth chasing.
Ref 39:
Ref 39:
does not, as far as I can make out, in any way support the statement it is purported to. The study wasn't designed to do so and I can only hope it was a typo when it was cited.
Ref 40 is gold dust
because it says this:
"In one study in vivo , subjects were given isotopically labelled fatty acids, and biopsies of different depots were taken at abdominal surgery 24 h later (Marin et al. 1992); accumulation of label was most marked (per g TG) in the omental and retroperitoneal depots."
"In one study in vivo , subjects were given isotopically labelled fatty acids, and biopsies of different depots were taken at abdominal surgery 24 h later (Marin et al. 1992); accumulation of label was most marked (per g TG) in the omental and retroperitoneal depots."
which allows us to find Marin et al's paper
The Morphology and Metabolism of Intraabdominal Adipose Tissue in Men
The Morphology and Metabolism of Intraabdominal Adipose Tissue in Men
which used real live humans in a tracer study coupled with adipose tissue biopsies during abdominal surgery. My sort of study. Just from the abstract we have, in-vivo:
"Adipose tissue lipid uptake, measured after oral administration of labeled oleic acid in triglyceride, was approximately 50% higher in omental than in subcutaneous adipose tissues."
and in vitro
"Adipocytes from omental fat also showed a higher lipolytic sensitivity and responsiveness to catecholamines"
So, easy in via insulin, easy out via sympathetic nervous system stimulation.
But also:
"Furthermore, these adipocytes were less sensitive to the antilipolytic effects of insulin."
"Furthermore, these adipocytes were less sensitive to the antilipolytic effects of insulin."
which is illustrated like this
Here we have visceral adipocytes as black circles and SC adipocytes as open circles. It's true that visceral adipocytes exposed to noradrenalin at 10⁻⁴ mmol/l can suppress this lipolysis less effectively than SC adipocytes do but the difference is relatively small compared to the very marked extra lipolysis under noradrenalin exposure in visceral adipocytes. Visceral adipocytes *should* stay small by balancing insulin signalling against sympathetic lipolysis.
So let's summarise:
Visceral/omental adipocytes are exquisitely insulin sensitive.
They perform markedly augmented lipolysis under sympathetic stimulation.
They have mildly impaired insulin suppression of sympathetic lipolysis.
I think that sets us up to think in sensible terms about what visceral fat does and why it is associated with metabolic syndrome.
Peter