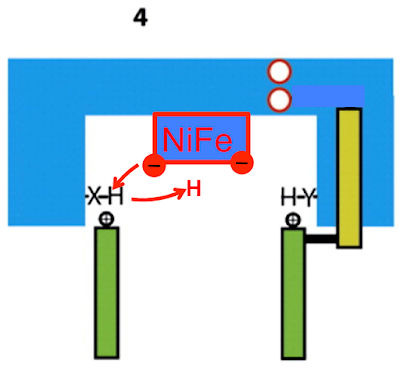
Just a quick run down of a possible mechanism of the membrane bound NiFe hydrogenases taken from the scheme for complex I function as suggested:
"A similar scheme can be used for the mechanism of NiFe-hydrogenases, where charge variations in the NiFe site drive conformational changes resulting in proton translocation."
quoted from
The coupling mechanism of respiratory complex I — A structural and evolutionary perspective
which was the basis of the last post. The modern NiFe system is pretty much the same as the CoQ system but it needs a simple summary here to allow us to try and run it in reverse, more in the LUCA style, and to try and to try and see what it might have been doing before it converted to a pump.Here we are, ready to start. These electrons have come from ferredoxin rather than NADH:
The electrons transfer from the FeS clusters to the NiFe cluster and the repulsive effect shown in the purple arrows disappears:
and is replaced by the large purple arrows signifying marked attraction:
and subsequent conformation change and ion pumping:
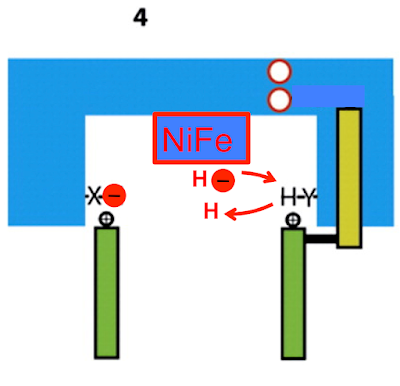
Next the two electrons have to be transfered to two protons to yield the molecular hydrogen which is the final product, comparable to the two hydrogens which exit on CoQ2H. This has mostly been studied in the soluble NiFe hydrogenases. The mechanism will be conserved and consensus suggests that it will involve an hydride ion, H-, attached to the Ni atom.
So we can start like this
which will rearrange to this:
giving us the hydride intermediate before exchange of the electron and acquisition of a proton from the amino acid Y:
to give us molecular hydrogen
To get us back to the start position we need, as per complex I/CoQ2H function, to supply of a pair of electrons and a pair of protons:
and we're good to go again:
That will do for to today. Obviously there is perhaps less interest in how anaerobic hydrogen evolving bacteria and archaea pump either protons or Na+ ions at the most frugal limits of bio-energetics when compared to complex I. So detailed work on the reverse process, where hydrogen powers a proton or Na+ gradient, is thin on the ground but this is where we need to look to see how a pH driven system for carbon fixation/ferredoxin generation might be converted to a system to preserve the energy derived from hydrogen as a proton/Na+ gradient which is a pre requisite to leaving the vent system as an independent organism.
Peter