Those adipose tissue TFAM knockout mice need a revisit. They have an engineered, adipose tissue specific, catastrophic injury to complex I. Complex I failure = insulin-independent DNL, so they should be fat. But they are thin, euglycaemic and have excellent glucose tolerance on a GTT. If complex I failure gives obesity, what is going on here?
These mice were one of the core triggers to me for the concept of TCA halting due to the inability of an injured complex I to oxidise NADH. It's very clear that rotenone, at concentrations which inhibit complex I without killing your model, activates de novo lipogenesis as a technique to deal with excess NADH, excess acetyl-CoA and to provide long chain saturated fatty acids to input as FADH2 to the ETC (at ETFdh) and so to run the rest of the ETC downstream of complex I.
Look at the ability of TFAM adipocytes to uptake 2-deoxyglucose with and without insulin. This is what you get:
TFAM mouse adipocytes appear to be VERY insulin sensitive. In fact they can uptake glucose in the complete absence of any insulin, at the same rate that control (Lox) adipocytes can under supra maximal* insulin. They are insulin hypersensitive without needing any insulin...
*In the supplementary methods these adipocytes are treated with 1.25iu/kg to get this graph, but they are in cell suspension after removal and predigestion, so I don't quite see what concentration of insulin was used. I think is a reasonable assumption that the concentration would be supra maximal...
Yet these adipocytes have mitochondria with a delta psi which is profoundly depressed. The photomicrographs show them as being completely f*cked. So enhanced insulin sensitivity is completely counterintuitive.
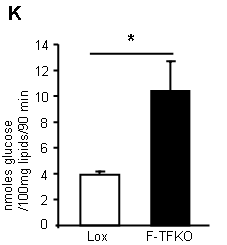
What do they do with the glucose they so readily take up? They convert it in to lipid (supplementary data Fig2):
*primary isolated adipocytes, real functional cells, supplied with 5mmol/l glucose.
This lipogenesis looks remarkably like the lipogenesis you see in tissue culture cells treated with rotenone (aside; or in post-obese humans during an OGTT). These cells are energy deprived through the inability to utilise NADH. They side step this by converting acetyl-CoA to lipid and then oxidise this lipid through beta oxidation, which is less dependent on the TCA. The cells appear to be insulin sensitive because they are starving. Even without insulin there is a huge glucose uptake. Once given a few GLUT4s by supplying insulin you get supra-huge glucose uptake. I have to wonder if this is simply a concentration gradient effect, hard to say from the paper. The actual number of GLUT4s (up or down in number cf controls) is completely model dependent, if you read around. But the glucose gets converted to lipid. When you look at ATP turnover you see starvation:
*Note, cell culture 3T3-L1 derivatives at 25mmol/l glucose.
Careful here, we have gone from real mice with TFAM KO adipocytes to TFAM suppressed cell lines. Cultured shTf1 have mild knockdown, shTf2 have severe. These are tissue culture cells. They may or may not behave in quite the same manner as cells from a real live slightly broken mouse...
But the message appears to be that the processes to extract energy from glucose need to be up-regulated to the maximum possible.
Back to real broken mice. Their adipocyte mitochondria are uncoupled (i.e. they fail to correctly increase delta psi when ATP-synthase is blocked by oligomycin) and have an elevated oxygen consumption rate when running on succinate (to bypass complex I)
The uncoupling is very interesting. My feeling is that these cells really are uncoupled, really are insulin resistant due to this and that whatever effect insulin has at high doses is taken advantage of, to the maximum amount possible. Hence the term "apparently" insulin sensitive, when actually insulin resistant...
You could suggest that the underlying insulin resistance is reflected in the glycerol release. These cells, which do insulin-independent glucose uptake, also release glycerol in larger amounts than controls. Oh, and look at the palmitate oxidation too:
The FFAs released from their glycerol backbone do not appear to be discharged in to the systemic circulation (in these mice), they get oxidised because the adipocytes are in ATP starvation and can still use ETFdh to generate some ATP. We have considered FFAs and uncoupling before. The ATP situation may not suggest uncoupling as a particularly good idea but FFAs are FFAs and, if insulin is silenced, then uncoupling seems unavoidable.
BTW the control of uncoupling is so fascinating we'll have to come back to it some other time. Needless to say it integrates ADP, ATP, CoQ redox status, cytoplasm:mitochonrial ATP ratio. And free fatty acid availability, of course.
So let's summarise: Complex I damaged adipocytes are greedy for glucose without (or with) insulin. They are concurrently insulin resistant, which limits their ability to store triglycerides. Their size is immaterial. I was driven to this by a paper which demonstrated that adipocytes isolated from sucrose/lard fed mice are all equally insulin resistant, irrespective of their size (what exactly determines basal lipolysis in an interesting question, I'd guess it is not simply size, though it must be related. It's probably very important). Obviously isolated adipocytes are clear of the influence of leptin, the ventromedial hypothalamus and the sympathetic nervous system, which will certainly not be the case in any intact mouse. But, at the level of very core metabolism, this makes sense in the light of macroscopic observations. When adipocytes STOP getting fatter they do so because complex I has broken to an adequate degree. As they cease to enlarge and cease to respond to insulin they release FFAs, without any concern for higher level metabolic signalling from insulin. Systemic elevated FFAs uncouple the rest of the body's mitochondria (outside the CNS) and on we go through IGT to diabetes.
Peter
Sunday, February 16, 2014
Monday, February 03, 2014
Dr Ravnskov on statins for primary prevention of CVD
This is more of a Facebook link than a blog post, but hats off to the BMJ as they have recently published some excellent articles in which neat truth appears to be very controversial (if you pedal b*ll*cks for a living).
Now they have published Dr Ravnskov's response to Ebrahim's pro statin for primary prevention paper. It's a nice reply and it's great to see a medical journal giving a voice to sanity. Of course, hats off to Dr R for being that voice.
You can read the letter here.
Peter
Now they have published Dr Ravnskov's response to Ebrahim's pro statin for primary prevention paper. It's a nice reply and it's great to see a medical journal giving a voice to sanity. Of course, hats off to Dr R for being that voice.
You can read the letter here.
Peter
Friday, January 31, 2014
TV Pantomine or the Oxford study
I don't ever watch television (we have no digital decoding box, the TV is for DVDs) and I can't waste the time to watch this performance on the internet.
There are two truisms I love.
First: How can you tell when a politician is lying? You see their lips move.
Equally good: Any semblance of television documentaries to real life is purely accidental.
For a lesson in how to stack a real study against low carb this Oxford group beats any television entertainment hands down. Also, if you ignore the crap from the researchers, there are lots of interesting (pro LC) data in this study, especially the myocardial energetics and body composition.
What they found, and which made the tittle of the paper, was the gem that LCHF eating "impairs cardiac high-energy phosphate metabolism". Baaaad?
What I really liked was that despite "9% lower cardiac PCr/ATP (P< 0.01)" there was "no change in cardiac function".
Ketones. Ketones allow normal cardiac function at reduced PCr/ATP levels. Ketones (from Veech's work) bypass insulin resistance, be that pathological as in Alzheimers or physiological as in very LCHF eating. Cardiac muscle functions well at reduced ATP levels, provided ketones are available.
Feeling like crap in the early stages of ketosis (Atkins Flu™) is common, it's usually at its absolute worst at about a week in to ketogenic eating. This group clearly knew how to time their cognitive testing! They have more of an agenda than I do.
Both the study and the TV drama have the potential to injure people who need ketogenic eating, I guess the TV show more so than the Oxford study, because it is more likely to stop grass roots level defections from Weight Watchers to genuinely healthy fat-based eating.
Peter
There are two truisms I love.
First: How can you tell when a politician is lying? You see their lips move.
Equally good: Any semblance of television documentaries to real life is purely accidental.
For a lesson in how to stack a real study against low carb this Oxford group beats any television entertainment hands down. Also, if you ignore the crap from the researchers, there are lots of interesting (pro LC) data in this study, especially the myocardial energetics and body composition.
What they found, and which made the tittle of the paper, was the gem that LCHF eating "impairs cardiac high-energy phosphate metabolism". Baaaad?
What I really liked was that despite "9% lower cardiac PCr/ATP (P< 0.01)" there was "no change in cardiac function".
Ketones. Ketones allow normal cardiac function at reduced PCr/ATP levels. Ketones (from Veech's work) bypass insulin resistance, be that pathological as in Alzheimers or physiological as in very LCHF eating. Cardiac muscle functions well at reduced ATP levels, provided ketones are available.
Feeling like crap in the early stages of ketosis (Atkins Flu™) is common, it's usually at its absolute worst at about a week in to ketogenic eating. This group clearly knew how to time their cognitive testing! They have more of an agenda than I do.
Both the study and the TV drama have the potential to injure people who need ketogenic eating, I guess the TV show more so than the Oxford study, because it is more likely to stop grass roots level defections from Weight Watchers to genuinely healthy fat-based eating.
Peter
Saturday, January 25, 2014
An aside on psychiatric links from Sid Dishes
Sid has recently put these two excellent links up on her Facebook timeline. The Protons thread is slowly working its way towards probable causes of complex I failure and an awful lot of the basic mitochondrial information comes from papers on Parkinsons disease or Alzheimers disease.
It has been clear for some time that many, many mitochondrial problems, when localised in specific sets of neurons, are categorised as psychiatric illnesses without needing to have a full blown genetic mitochondrial disease at their core. Acquired mitochondrial dysfunction, most likely at complex I, might well be all you need. Have the right SNPs in genes for proteins of the ETC or assorted ion channels might allow you to be allocated schizophrenia, bipolar disorder or major depression as your pigeonhole.
Trying to treating mitochondrial psychosis by tinkering with the superficial knock on effects at the neurotransmitter level will be of limited effectiveness. Altering bioenergetics using an NAD+ precursor or by inducing ketosis might be far more logical approaches.
Enjoy:
The psychiatric presentation of mitochondrial disorders in adults
Nicotinamide, NAD(P)(H), and Methyl-Group Homeostasis Evolved and Became a Determinant of Ageing Diseases: Hypotheses and Lessons from Pellagra.
The second paper is a rather broad brushed picture of evolution, society and NADH. Gross NAD deficiency with adequate calories (pellagra) will mean that there is almost no time for NAD+ to exist before being reconverted to NADH. The high NADH/NAD+ ratio will particularly favour excess superoxide generation at complex I if there is any reverse electron flow from the CoQ couple. It's a pity this paper does't have mtG3Pdh in its diagram of the ETC. An interesting read even if it's full of concepts which the Hyperlipid perspective might question or might invert the causality there-of.
EDIT: I found this one myself and nearly lost it. The perils of PubMed-ing in your lunch break at work and not emailing the link to yourself!
Neuroanatomical Pattern of Mitochondrial Complex I Pathology Varies between Schizophrenia, Bipolar Disorder and Major Depression
END EDIT
Peter
It has been clear for some time that many, many mitochondrial problems, when localised in specific sets of neurons, are categorised as psychiatric illnesses without needing to have a full blown genetic mitochondrial disease at their core. Acquired mitochondrial dysfunction, most likely at complex I, might well be all you need. Have the right SNPs in genes for proteins of the ETC or assorted ion channels might allow you to be allocated schizophrenia, bipolar disorder or major depression as your pigeonhole.
Trying to treating mitochondrial psychosis by tinkering with the superficial knock on effects at the neurotransmitter level will be of limited effectiveness. Altering bioenergetics using an NAD+ precursor or by inducing ketosis might be far more logical approaches.
Enjoy:
The psychiatric presentation of mitochondrial disorders in adults
Nicotinamide, NAD(P)(H), and Methyl-Group Homeostasis Evolved and Became a Determinant of Ageing Diseases: Hypotheses and Lessons from Pellagra.
The second paper is a rather broad brushed picture of evolution, society and NADH. Gross NAD deficiency with adequate calories (pellagra) will mean that there is almost no time for NAD+ to exist before being reconverted to NADH. The high NADH/NAD+ ratio will particularly favour excess superoxide generation at complex I if there is any reverse electron flow from the CoQ couple. It's a pity this paper does't have mtG3Pdh in its diagram of the ETC. An interesting read even if it's full of concepts which the Hyperlipid perspective might question or might invert the causality there-of.
EDIT: I found this one myself and nearly lost it. The perils of PubMed-ing in your lunch break at work and not emailing the link to yourself!
Neuroanatomical Pattern of Mitochondrial Complex I Pathology Varies between Schizophrenia, Bipolar Disorder and Major Depression
END EDIT
Peter
Thursday, January 23, 2014
Macrobiosis, macrobiopathy?
I hope we all remember Barnad's low fat vegan treatment for diabetes. This figure sums it up:
By 74 weeks folks are not looking very well controlled. Certainly not compared to sustained LCHF. Now, if we crop it nicely we get:
Which makes low fat veganism look pretty good, just so long as you limit your study to 12 weeks.
How a bout a macrobiotic diet?
Very low fat, low protein, LOADS of fibre and complex grain based carbs.
Take some diabetics, measure some numbers, feed for three weeks by skilled macrobiotic cooks while teaching macrobiotic cookery, re test at twelve weeks after self preparing food at home for the last nine of those weeks.
Cured?
We don't get HbA1cs in this study but we have the fasting glucose levels, the 2h post prandial levels, the lipid levels and I quite like the blood pressure levels.
Everything improved when you have a real macrobiotic cook serving you. Do it yourself and by twelve weeks things are already starting to fall to pieces:
Two hour post prandial glucose levels are already rising by 3 months. Even Barnard's study preserved glycaemia for longer than this. You might expect triglycerides to be rising too. They are. If you could give a monkeys about cholesterol levels they too are deteriorating:
Blood pressures pre intervention 127/76, 3 weeks 113/69, 12 weeks, ohoh, 118/75.
Of course you could always claim that these folks weren't doing the macrobiotic diet correctly at home.
Me, I think they are metabolically broken and, even before they stop losing weight, they show their broken metabolism by their climbing post prandial (and fasting) glycaemia. By 74 weeks they will be worse off than Barnards failures.
There is no answer for diabetics other than LCHF.
Sometimes things come up in comments which are so good. As Johnny so kindly obliged the person who linked to the macrobiotic study in their request for comments:
"Yes I would like to comment. Please put your keyboard into a potato sack and throw it into the sewer"
Like.
Peter
BTW, if anyone can reconcile Fig 1 and the red line in Fig 2, congratulations!
By 74 weeks folks are not looking very well controlled. Certainly not compared to sustained LCHF. Now, if we crop it nicely we get:
Which makes low fat veganism look pretty good, just so long as you limit your study to 12 weeks.
How a bout a macrobiotic diet?
Very low fat, low protein, LOADS of fibre and complex grain based carbs.
Take some diabetics, measure some numbers, feed for three weeks by skilled macrobiotic cooks while teaching macrobiotic cookery, re test at twelve weeks after self preparing food at home for the last nine of those weeks.
Cured?
We don't get HbA1cs in this study but we have the fasting glucose levels, the 2h post prandial levels, the lipid levels and I quite like the blood pressure levels.
Everything improved when you have a real macrobiotic cook serving you. Do it yourself and by twelve weeks things are already starting to fall to pieces:
Two hour post prandial glucose levels are already rising by 3 months. Even Barnard's study preserved glycaemia for longer than this. You might expect triglycerides to be rising too. They are. If you could give a monkeys about cholesterol levels they too are deteriorating:
Blood pressures pre intervention 127/76, 3 weeks 113/69, 12 weeks, ohoh, 118/75.
Of course you could always claim that these folks weren't doing the macrobiotic diet correctly at home.
Me, I think they are metabolically broken and, even before they stop losing weight, they show their broken metabolism by their climbing post prandial (and fasting) glycaemia. By 74 weeks they will be worse off than Barnards failures.
There is no answer for diabetics other than LCHF.
Sometimes things come up in comments which are so good. As Johnny so kindly obliged the person who linked to the macrobiotic study in their request for comments:
"Yes I would like to comment. Please put your keyboard into a potato sack and throw it into the sewer"
Like.
Peter
BTW, if anyone can reconcile Fig 1 and the red line in Fig 2, congratulations!
Tuesday, January 21, 2014
I like this
From Phillip (Thanks):
Impaired glucose tolerance in low-carbohydrate diet: maybe only a physiological state.
Like, like, like, like, like, like, like, like, like, LIKE!
Peter
Sunday, January 19, 2014
Acipimox and insulin action
I just wanted to put up a brief mention of this paper on acipimox.
Acipimox is an inhibitor of lipolysis. It's essentially useless as a therapy for anything, partly because it is derived from an incorrect paradigm but mostly because it's impossible to get it to work for any extended period of time. It's good for a week though.
So let's take a few diabetics, do some lab work on them, drop their FFAs using acipimox, then repeat their lab work a week later.
Fasting FFAs drop from 563 micromol/l (not actually very high) to 230 micromol/l (verging on pathologically low) and FBG drops from 8.5mmol/l to 7.0mmol/l. All highly significant, statistically.
Does this mean that they are fixed, i.e. they can go out and eat pizza all day and be normoglycaemic?
No.
A diabetic person who drinks 75g of glucose in water will hit a 2h blood glucose of about 16mmol/l. With markedly reduced FFAs they will be graced with a 2h blood glucose of a mere 14mmol/l, which does not look like dropping. They're f*cked, metabolically. Last time I did this (2008ish??) my 2h BG was 3.4mmol/l.
It looks to me as if acipimox removes the normal physiological uncoupling associated with abnormally elevated FFAs and leaves the insulin resistance of a broken set of mitochondria there for all to see.
There is physiological insulin resistance associated with elevated FFAs. Then there is pathological insulin resistance from mitochondrial dysfunction.
Just wanted to say...
Peter
Acipimox is an inhibitor of lipolysis. It's essentially useless as a therapy for anything, partly because it is derived from an incorrect paradigm but mostly because it's impossible to get it to work for any extended period of time. It's good for a week though.
So let's take a few diabetics, do some lab work on them, drop their FFAs using acipimox, then repeat their lab work a week later.
Fasting FFAs drop from 563 micromol/l (not actually very high) to 230 micromol/l (verging on pathologically low) and FBG drops from 8.5mmol/l to 7.0mmol/l. All highly significant, statistically.
Does this mean that they are fixed, i.e. they can go out and eat pizza all day and be normoglycaemic?
No.
A diabetic person who drinks 75g of glucose in water will hit a 2h blood glucose of about 16mmol/l. With markedly reduced FFAs they will be graced with a 2h blood glucose of a mere 14mmol/l, which does not look like dropping. They're f*cked, metabolically. Last time I did this (2008ish??) my 2h BG was 3.4mmol/l.
It looks to me as if acipimox removes the normal physiological uncoupling associated with abnormally elevated FFAs and leaves the insulin resistance of a broken set of mitochondria there for all to see.
There is physiological insulin resistance associated with elevated FFAs. Then there is pathological insulin resistance from mitochondrial dysfunction.
Just wanted to say...
Peter
Thursday, January 16, 2014
Protons (34) Rotenone
So far I've been thinking about insulin resistance as a normal physiological process for regulating the energy input in to each cell, on an individual metabolic needs basis. There is nothing pathological about this process, regulation is essential. To develop insulin resistance in the immediate aftermath of a single 2000kcal meal or during a 5 day fast is exactly what you need to do. Combining the post prandial state with the fasting state is pathological but could be corrected by simply restoring insulin sensitivity to adipocytes, so allowing them to lower free fatty acids when glucose is elevated.
There are times when I would like there to be differing terms for the insulin resistance of the immediate post prandial period, of starvation and of metabolic broken-ness. It would make for a much clearer picture than saying someone is "insulin resistant".
Now I'd like to think about some pathology.
I’m going to start with this paper on rotenone, with thanks to Mike for the full text. I'm not sure how widely rotenone is used nowadays but, in the lab, it is a freely available inhibitor of complex I. I doubt this toxicosis is particularly prevalent in everyday life, I'm more interested in a basic mechanism which we can extend to other injuries of complex I. Adding rotenone to almost any cell line produces this sort of effect on metabolism, here in C2C12 muscle-like cells (which do it best). From Fig4:
Oxygen consumption is depressed, delta psi is depressed and NADH accumulates at the expense of lowered NAD+ levels. BTW, some authors use the NADH/NAD+ ratio, some the inverse. Ah well, it's still badness. These folks talk about "reductive stress".
The interesting point to note from section D is the rise in acetyl-carnitine, a molecule used to export acetyl-CoA from mitochondria to cytoplasm.
If we block the ability of the mitochondria to feed NADH in to the ETC there is no point in turning the TCA because this generates four NADHs for that single FADH2 from succinate dehydrogenase (which still works if you provide succinate exogenously). Instead the acetyl-CoA is exported as acetyl-carninitine or as citrate after combination with oxaloacetate. Once in to the cytoplasm acetyl-CoA is available for de novo lipogenesis. Does it get used for this?
Here are some C2C12 muscle-like cells which are running on a "low" concentration (probably around 5.0mmol/l) of glucose. The ones on the right are also exposed to 5.0 nanomol of rotenone in the complete absence of insulin or any equivalent. Cytoplasm is pale purple, nucleus is dark purple:

The beautiful orange-red staining droplets are lipid, more clearly visible in this enlargement:

How much lipid is deposited? That depends on how much rotenone is used and how long the complex I has been blocked for. From Fig 2:
You can do exactly the same thing by blocking complex I with piericidin A or by knocking down expression of the gene NDUFV1 (codes for a huge chunk of complex I) in C2C12 cells. From Fig 3:
You get the same effect in people with the misfortune to be born with severe defects in complex I; in the affected tissues there is lipid accumulation. By the time you get down to <5% complex one activity there are quite nasty knock on effects on fatty acid oxidation and cell viability, but that's another story.
Very few people would choose to take rotenone nowadays but there are a number of other complex I inhibitors, some of which are quite widely available. Flunarizine, isoniazid and atrazine spring to mind with bisphenyl A as a more general mitochondrial toxin which includes complex I blockade. None are marketed as weight loss supplements.
I see this as a generic mechanism. Complex I dysfunction leads to intracellular lipid accumulation.
What else is interesting?
These cells are being fed with low levels of glucose without insulin. Glucose uptake will be basal and much of the energy from glycolysis will be diverted to lipogenesis. Again, if ox phos is reduced mitochondrial ATP production will be depressed and glycolysis will be the main source of ATP using substrate level phosphorylation. We know lipogenesis is occurring, so we must be deriving NADPH from glycolysis. If we were to add insulin to the culture medium of a rotenone poisoned cell and perhaps increase the glucose level to 25mmol/l, what would happen?
Assuming we can generate enough of a delta psi to allow insulin signalling we would pour glucose in to the cell and down through glycolysis to pyruvate. This gives us a ton of acetyl-CoA in the mitochondria. Complex I is still blocked. What's a cell to do except de novo lipogenesis in its cytoplasm?
As we allow glucose in to a cell, we push de novo lipogenesis. This is a parody of insulin sensitivity. The faster we allow glucose in to a cell, the faster it is lost to lipid. My line of thought is about how well this ability to sequester glucose as lipid might give the impression of being extremely sensitive to insulin, when the actual mitochondria are dysfunctional and should be signalling insulin resistance.
I can't get away from the unarguable fact that post-obese women perform enough DNL to get their RQ > 1.0 on exposure to 75g of glucose in an OGTT.
Perhaps we need to look at the mitochondrial function of people who's "preferred" metabolic state is best achieved by obesity. And how we might damage complex I without mainlining rotenone.
Better hit "post" as things keep getting in the way of extending this particular entry.
Peter
There are times when I would like there to be differing terms for the insulin resistance of the immediate post prandial period, of starvation and of metabolic broken-ness. It would make for a much clearer picture than saying someone is "insulin resistant".
Now I'd like to think about some pathology.
I’m going to start with this paper on rotenone, with thanks to Mike for the full text. I'm not sure how widely rotenone is used nowadays but, in the lab, it is a freely available inhibitor of complex I. I doubt this toxicosis is particularly prevalent in everyday life, I'm more interested in a basic mechanism which we can extend to other injuries of complex I. Adding rotenone to almost any cell line produces this sort of effect on metabolism, here in C2C12 muscle-like cells (which do it best). From Fig4:
Oxygen consumption is depressed, delta psi is depressed and NADH accumulates at the expense of lowered NAD+ levels. BTW, some authors use the NADH/NAD+ ratio, some the inverse. Ah well, it's still badness. These folks talk about "reductive stress".
The interesting point to note from section D is the rise in acetyl-carnitine, a molecule used to export acetyl-CoA from mitochondria to cytoplasm.
If we block the ability of the mitochondria to feed NADH in to the ETC there is no point in turning the TCA because this generates four NADHs for that single FADH2 from succinate dehydrogenase (which still works if you provide succinate exogenously). Instead the acetyl-CoA is exported as acetyl-carninitine or as citrate after combination with oxaloacetate. Once in to the cytoplasm acetyl-CoA is available for de novo lipogenesis. Does it get used for this?
Here are some C2C12 muscle-like cells which are running on a "low" concentration (probably around 5.0mmol/l) of glucose. The ones on the right are also exposed to 5.0 nanomol of rotenone in the complete absence of insulin or any equivalent. Cytoplasm is pale purple, nucleus is dark purple:

The beautiful orange-red staining droplets are lipid, more clearly visible in this enlargement:

How much lipid is deposited? That depends on how much rotenone is used and how long the complex I has been blocked for. From Fig 2:
You can do exactly the same thing by blocking complex I with piericidin A or by knocking down expression of the gene NDUFV1 (codes for a huge chunk of complex I) in C2C12 cells. From Fig 3:
You get the same effect in people with the misfortune to be born with severe defects in complex I; in the affected tissues there is lipid accumulation. By the time you get down to <5% complex one activity there are quite nasty knock on effects on fatty acid oxidation and cell viability, but that's another story.
Very few people would choose to take rotenone nowadays but there are a number of other complex I inhibitors, some of which are quite widely available. Flunarizine, isoniazid and atrazine spring to mind with bisphenyl A as a more general mitochondrial toxin which includes complex I blockade. None are marketed as weight loss supplements.
I see this as a generic mechanism. Complex I dysfunction leads to intracellular lipid accumulation.
What else is interesting?
These cells are being fed with low levels of glucose without insulin. Glucose uptake will be basal and much of the energy from glycolysis will be diverted to lipogenesis. Again, if ox phos is reduced mitochondrial ATP production will be depressed and glycolysis will be the main source of ATP using substrate level phosphorylation. We know lipogenesis is occurring, so we must be deriving NADPH from glycolysis. If we were to add insulin to the culture medium of a rotenone poisoned cell and perhaps increase the glucose level to 25mmol/l, what would happen?
Assuming we can generate enough of a delta psi to allow insulin signalling we would pour glucose in to the cell and down through glycolysis to pyruvate. This gives us a ton of acetyl-CoA in the mitochondria. Complex I is still blocked. What's a cell to do except de novo lipogenesis in its cytoplasm?
As we allow glucose in to a cell, we push de novo lipogenesis. This is a parody of insulin sensitivity. The faster we allow glucose in to a cell, the faster it is lost to lipid. My line of thought is about how well this ability to sequester glucose as lipid might give the impression of being extremely sensitive to insulin, when the actual mitochondria are dysfunctional and should be signalling insulin resistance.
I can't get away from the unarguable fact that post-obese women perform enough DNL to get their RQ > 1.0 on exposure to 75g of glucose in an OGTT.
Perhaps we need to look at the mitochondrial function of people who's "preferred" metabolic state is best achieved by obesity. And how we might damage complex I without mainlining rotenone.
Better hit "post" as things keep getting in the way of extending this particular entry.
Peter
Sunday, December 29, 2013
Protons (33) The mtG3Pdh knockout mice
These mice keep asking me more questions that I'm comfortable with so the post is down subject to further thinking!
Time for a Christmas break methinks! Best wishes all round.
Peter
OK, I've stopped trying to cover every aspect of these mice, some of the more exciting bits really need a whole series on their own. So here we have the slightly truncated version of the original post.
Repost.
Before we come to the consequences of excessive superoxide generation in complex I I'd just like to run through a small part of this rather neat paper on what happens if you virtually eliminate it.
Take a mtG3Pdh knockout mouse and feed it on a very low fat standard style lab chow and it will grow pretty well normally. Bear in mind that both of the diets in the study are very, very low in fat.
Ultra low fat means that dietary fat, especially when PUFA based, is not going to generate any insulin resistance based on electron transferring flavoprotein dehydrogenase reducing the CoQ couple. It's going to have to be mtG3Pdh which controls insulin facilitated calorie delivery. This particular model may not behave as simply on a higher fat diet. There are stacks of unknowns about this model.
Before we come on to anything else, what happens when you inject any old mouse with exogenous insulin?
Simple so long as you have the dose rate carefully chosen, blood glucose falls, cells develop insulin-induced insulin resistance, mouse survives. You have to be modest in your dose but it's not too difficult. We talked extensively about the Somogyi effect back in the Zombie days.
The mice in the present study happen to have been fed a high sucrose/minimal fat diet for 6 months. The two top lines are the wild type and heterozygous knockouts for mtG3Pdh. The lower, terminated, line represents the full knockouts.
The line is terminated at around 30 minutes because all of the mice were terminated, ie they died of hypoglycaemia. They needed to develop insulin-induced insulin resistance and, without mtG3Pdh, it never came. RIP.
The paper also goes on to show many, many other fascinating things about these mice but the more I try to cover all of these features the more it obscures the main finding. For insulin resistance on a ultra low fat diet you need mtG3Pdh up and running. It may be worth coming back to the effects of fructose and oxygen consumption from this paper but that's another set of ideas.
That's all for today. I just wanted to link these mice to the normal physiological insulin resistance generated by mtG3Pdh (when glucose/fructose is the primary metabolic fuel) and the protons ideas. They all fit together.
Core message: For hyperglycaemia, exogenous insulin or fructose to induce insulin resistance you need mtG3Pdh for effective reverse electron flow through complex I to generate (essential) superoxide/H2O2. This is important. So is going over the top with this normal physiological function, which looks to be rather bad for you.
Peter
Time for a Christmas break methinks! Best wishes all round.
Peter
OK, I've stopped trying to cover every aspect of these mice, some of the more exciting bits really need a whole series on their own. So here we have the slightly truncated version of the original post.
Repost.
Before we come to the consequences of excessive superoxide generation in complex I I'd just like to run through a small part of this rather neat paper on what happens if you virtually eliminate it.
Take a mtG3Pdh knockout mouse and feed it on a very low fat standard style lab chow and it will grow pretty well normally. Bear in mind that both of the diets in the study are very, very low in fat.
Ultra low fat means that dietary fat, especially when PUFA based, is not going to generate any insulin resistance based on electron transferring flavoprotein dehydrogenase reducing the CoQ couple. It's going to have to be mtG3Pdh which controls insulin facilitated calorie delivery. This particular model may not behave as simply on a higher fat diet. There are stacks of unknowns about this model.
Before we come on to anything else, what happens when you inject any old mouse with exogenous insulin?
Simple so long as you have the dose rate carefully chosen, blood glucose falls, cells develop insulin-induced insulin resistance, mouse survives. You have to be modest in your dose but it's not too difficult. We talked extensively about the Somogyi effect back in the Zombie days.
The mice in the present study happen to have been fed a high sucrose/minimal fat diet for 6 months. The two top lines are the wild type and heterozygous knockouts for mtG3Pdh. The lower, terminated, line represents the full knockouts.
The line is terminated at around 30 minutes because all of the mice were terminated, ie they died of hypoglycaemia. They needed to develop insulin-induced insulin resistance and, without mtG3Pdh, it never came. RIP.
The paper also goes on to show many, many other fascinating things about these mice but the more I try to cover all of these features the more it obscures the main finding. For insulin resistance on a ultra low fat diet you need mtG3Pdh up and running. It may be worth coming back to the effects of fructose and oxygen consumption from this paper but that's another set of ideas.
That's all for today. I just wanted to link these mice to the normal physiological insulin resistance generated by mtG3Pdh (when glucose/fructose is the primary metabolic fuel) and the protons ideas. They all fit together.
Core message: For hyperglycaemia, exogenous insulin or fructose to induce insulin resistance you need mtG3Pdh for effective reverse electron flow through complex I to generate (essential) superoxide/H2O2. This is important. So is going over the top with this normal physiological function, which looks to be rather bad for you.
Peter
Tuesday, December 24, 2013
Protons (32) Post obese insulin induced thermogenesis
Post-obese people are probably rarer than pre-obese people but at least they can be quite conclusively identified. The main problem with most post-obese folks is that they are usually only ephemerally in that state and they rarely achieve a truly "normal" bodyweight. But there are some people out there who have done this. So how do conventional medics get obese people to lose 80% of their excess bodyweight and keep that weight off for more than two years?
Well, it's quite easy. You just rearrange their digestive system to virtually join their stomach to their colon. Tatarinni wrote the paper. Eat, have a bowel movement, eat some more, poo some more. Maybe you have to eat sitting on the loo. Once patients have "adapted" to their bilio-pancreatic diversion they can get down to as few as 3-5 bowel movements a day, allowing them to leave the bathroom occasionally.
This is what you do (I added the red arrow for clarity):
Total remaining absorptive gut is about 250cm long. This works for weight loss. Tataranni's paper is fascinating as it gives us a picture of the metabolism of eight post-morbidly-obese women who are close to an ideal BMI and who have been that way for over two years.
You need the caveat that these people have a markedly maligned digestive system, so may not represent their metabolic state pre-obesity, but they are very interesting never the less. You also could make an argument that these people, given a normal digestive system, would rapidly become obese again. So perhaps they may really tell us something about people who are pre-obese.
The most striking aspect is that they are NOT insulin resistant. Fasting insulin and fasting glucose are quite, quite normal. Their resting metabolic rate is indistinguishable from that of control women.
But they are not quite normal. The response to a 75g oral glucose load shows markedly increased insulin sensitivity.
Let's just emphasise: Post-obese women with long term sustained normal bodyweight have a significantly increased sensitivity to insulin during an OGTT compared with never-obese women.
Fasting free fatty acids are lower, as you might expect, albeit ns in a group size of eight.
Obviously, with limited access to FFAs, the control of metabolic substrate supply at the cell surface must be managed by manipulating GLUT4s using a glycolysis derived input, which of course means mtG3Pdh as the CoQ input to resist insulin's action. This means there must be enhanced glucose (or insulin) induced thermogenesis to achieve this insulin resistance. Here it is in the aftermath of an OGTT:
The excess energy expenditure is, in part, heat generated by the in-putting of high energy NADH electrons to the CoQ couple without pumping protons.
I floated the concept that glycerol-3-phosphate might be a core protectant against caloric overload on an individual cell basis in the last post, by inducing insulin resistance. I also suggested that the other related function might be the diversion of excess calories to lipid storage, phosphorylated glycerol being essential for intracellular triglyceride formation.
So here we have another interesting set of graphs from Fig 5:
The first striking thing is that in post-obese people an oral load of 75g of glucose induces a respiratory quotient of greater than one. Second is that, during this time, lipid oxidation becomes negative. It was only ever half that of the never obese controls to begin with. As the authors comment:
"After the oral glucose load, the RQ increased more in P0 [post-obese] than in C [control] subjects, reaching values > 1. Thus, lipid synthesis exceeded lipid oxidation in P0 subjects 45 min after the oral glucose load and continued to do so for 40 more min".
What is happening is that these women accept glucose in to their cells very easily. The glucose is converted to pyruvate, this is decarboxylated via the pyruvate dehydrogenase complex to yield CO2 which increases the RQ. The acetyl CoA formed is exported to the cytoplasm as citrate. Obviously the citrate is formed by combining acetyl CoA with oxaloacetate, the latter can also be derived from pyruvate but this time via carboxylation, and hence the TCA never turns. Oxygen is never consumed. RQ >1.0.
So these post-obese women are exquisitely sensitive to insulin, in particular they are remarkably efficient at de novo lipogenesis and at the inhibition of lipolysis.
Were they like this before becoming obese? I think so. Why they might be like this is interesting to think about from the mitochondrial point of view.
Might there be any way of controling their weigh gain without the need for gross malabsorption secondary to removing most of their gut?
Well, you could take insulin out of the equation by simple ketogenic eating and see what happens...
I was going to leave this as an interesting snippet but there are a few add-ons to these ideas.
Some artificial models of this effect are available from various "pre-obese" rodent models. I had a think about them here.
Edward emailed me a link to this paper about a post-obese case report from Dundee. This man has a normal digestive system, he simply didn't use it for 382 days.
Look at the glucose levels in Table 1:
NB, these glucose levels are all very, very low. The authors feel that these values are real. Perhaps he may have been morbidly obese, yet still insulin sensitive. You need to have retained some insulin sensitivity to attain massive obesity without limiting weight gain by the transition to diabetes. But anyhoo, the trends are what interested me.
What we need to look at is the first column, fasting glucose levels. If we ignore day 355, where there was some sort of a hiccup, FBG was around 35mg/dl. This is quite low but the chap was in extended starvation so this might not be surprising. This is the level of glucose under deep, deep physiological insulin resistance. Ignore day seven value of re-feeding because metabolism will, in all probability, still be far from normal and the chap was only consuming liquid glucose at this time.
Instead I looked at day 55 of re-feeding, while he was on 1000kcal of a mixed diet. His FBG was very low, about two thirds of what it was during fasting. This chap, like the Italian enterectomy women, was very, very insulin sensitive. Insulin drives fat storage as well as hypoglycaemia.
He kept the weight off for at least 5 years. Two points: This chap was a psychological outlier! Second is that 1000kcal/d, if it is Food based, is a LC diet even if it is also a low-everything-else diet too.
Enjoy the winter festivities!
Peter
Well, it's quite easy. You just rearrange their digestive system to virtually join their stomach to their colon. Tatarinni wrote the paper. Eat, have a bowel movement, eat some more, poo some more. Maybe you have to eat sitting on the loo. Once patients have "adapted" to their bilio-pancreatic diversion they can get down to as few as 3-5 bowel movements a day, allowing them to leave the bathroom occasionally.
This is what you do (I added the red arrow for clarity):
Total remaining absorptive gut is about 250cm long. This works for weight loss. Tataranni's paper is fascinating as it gives us a picture of the metabolism of eight post-morbidly-obese women who are close to an ideal BMI and who have been that way for over two years.
You need the caveat that these people have a markedly maligned digestive system, so may not represent their metabolic state pre-obesity, but they are very interesting never the less. You also could make an argument that these people, given a normal digestive system, would rapidly become obese again. So perhaps they may really tell us something about people who are pre-obese.
The most striking aspect is that they are NOT insulin resistant. Fasting insulin and fasting glucose are quite, quite normal. Their resting metabolic rate is indistinguishable from that of control women.
But they are not quite normal. The response to a 75g oral glucose load shows markedly increased insulin sensitivity.
Let's just emphasise: Post-obese women with long term sustained normal bodyweight have a significantly increased sensitivity to insulin during an OGTT compared with never-obese women.
Fasting free fatty acids are lower, as you might expect, albeit ns in a group size of eight.
Obviously, with limited access to FFAs, the control of metabolic substrate supply at the cell surface must be managed by manipulating GLUT4s using a glycolysis derived input, which of course means mtG3Pdh as the CoQ input to resist insulin's action. This means there must be enhanced glucose (or insulin) induced thermogenesis to achieve this insulin resistance. Here it is in the aftermath of an OGTT:
The excess energy expenditure is, in part, heat generated by the in-putting of high energy NADH electrons to the CoQ couple without pumping protons.
I floated the concept that glycerol-3-phosphate might be a core protectant against caloric overload on an individual cell basis in the last post, by inducing insulin resistance. I also suggested that the other related function might be the diversion of excess calories to lipid storage, phosphorylated glycerol being essential for intracellular triglyceride formation.
So here we have another interesting set of graphs from Fig 5:
The first striking thing is that in post-obese people an oral load of 75g of glucose induces a respiratory quotient of greater than one. Second is that, during this time, lipid oxidation becomes negative. It was only ever half that of the never obese controls to begin with. As the authors comment:
"After the oral glucose load, the RQ increased more in P0 [post-obese] than in C [control] subjects, reaching values > 1. Thus, lipid synthesis exceeded lipid oxidation in P0 subjects 45 min after the oral glucose load and continued to do so for 40 more min".
What is happening is that these women accept glucose in to their cells very easily. The glucose is converted to pyruvate, this is decarboxylated via the pyruvate dehydrogenase complex to yield CO2 which increases the RQ. The acetyl CoA formed is exported to the cytoplasm as citrate. Obviously the citrate is formed by combining acetyl CoA with oxaloacetate, the latter can also be derived from pyruvate but this time via carboxylation, and hence the TCA never turns. Oxygen is never consumed. RQ >1.0.
So these post-obese women are exquisitely sensitive to insulin, in particular they are remarkably efficient at de novo lipogenesis and at the inhibition of lipolysis.
Were they like this before becoming obese? I think so. Why they might be like this is interesting to think about from the mitochondrial point of view.
Might there be any way of controling their weigh gain without the need for gross malabsorption secondary to removing most of their gut?
Well, you could take insulin out of the equation by simple ketogenic eating and see what happens...
I was going to leave this as an interesting snippet but there are a few add-ons to these ideas.
Some artificial models of this effect are available from various "pre-obese" rodent models. I had a think about them here.
Edward emailed me a link to this paper about a post-obese case report from Dundee. This man has a normal digestive system, he simply didn't use it for 382 days.
Look at the glucose levels in Table 1:
NB, these glucose levels are all very, very low. The authors feel that these values are real. Perhaps he may have been morbidly obese, yet still insulin sensitive. You need to have retained some insulin sensitivity to attain massive obesity without limiting weight gain by the transition to diabetes. But anyhoo, the trends are what interested me.
What we need to look at is the first column, fasting glucose levels. If we ignore day 355, where there was some sort of a hiccup, FBG was around 35mg/dl. This is quite low but the chap was in extended starvation so this might not be surprising. This is the level of glucose under deep, deep physiological insulin resistance. Ignore day seven value of re-feeding because metabolism will, in all probability, still be far from normal and the chap was only consuming liquid glucose at this time.
Instead I looked at day 55 of re-feeding, while he was on 1000kcal of a mixed diet. His FBG was very low, about two thirds of what it was during fasting. This chap, like the Italian enterectomy women, was very, very insulin sensitive. Insulin drives fat storage as well as hypoglycaemia.
He kept the weight off for at least 5 years. Two points: This chap was a psychological outlier! Second is that 1000kcal/d, if it is Food based, is a LC diet even if it is also a low-everything-else diet too.
Enjoy the winter festivities!
Peter
Saturday, December 21, 2013
Protons (31) insulin induced thermogenesis in the Pima
I thought I might just put this snippet up as it's been lying around ready to go for some time. I've tried to tidy up some of the worst sentences. Happy Solstice! Here we go.
Back when talking about the Pima paper I skipped over insulin induced thermogenesis, a fascinating subject and a phenomenon distinctly lacking in the obese, if they are insulin resistant. This is the pattern of IIT, from normal glucose tolerance through to diabetes. From Fig 2:
The phrase "insulin induced thermogenesis" is, at first glance, a complete oxymoron. It is difficult to imagine any way in which insulin, that most effective suppressor of free fatty acids, might uncouple respiration to generate heat. The primary brown adipose tissue technique is to use UCP1, which depends on free fatty acid availability. But there are other ways to generate heat in addition to uncoupling the proton gradient. It is worth noting that some of the thermogenesis is "obligatory", by which the authors mean the exothermic storage of glucose as glycogen. I'm rather more interested in the "facultative" component.
Brand's group have been hard at work again and have this (excellent) paper out:
Sites of reactive oxygen species generation by mitochondria oxidizing different substrates
most especially the lovely figure 1 (use the link to get the legend, it's a good summary of ETC energetics and "non-stressed" superoxide production):
It is an abstraction of the ETC, grouping inputs by their redox potential rather than physical location and is now becoming pretty inclusive for most electron donors. All mitochondrial NADH inputs are through complex I at -280mV and these electrons flow from here towards the positive potential of +600mV, provided by oxygen at complex IV. They do work in the process, pumping protons to set up the delta psi which can be "wasted" to generate heat when needed. Without uncoupling, the process is efficient and energy from the electron is largely conserved.
The interesting point is that mtG3Pdh inputs to the CoQ couple which has, we can now see, a redox potential of +20mV.
Specifically, mtG3Pdh is taking a cytoplasmic NADH, which could theoretically be shuttled to the mitochondrial matrix, and hence to complex I, at -280mV, and inputting it to the ETC at +20mV. The energy lost by skipping from -280mV to +20mV would normally pump four protons and now appears as heat.
After a glucose load any oxidation of the abnormally elevated FFAs of insulin resistant people still provides a continuous +20mV input using ETF dehydrogenase's FADH2 acting on the CoQ couple, which is ultimately derived from the FADH2 of the first step of beta oxidation, ie it's FADH2 transporting electrons all the way, there is no energy wastage when using fats to limit insulin's action. In the absence of these inappropriate FFAs, the correct way to reduce the CoQ couple is using mtG3Pdh which shuts down insulin's action at the cost of generating heat because it uses NADH, stepped down to FADH2, as a direct input at the CoQ level. We want a reduced the CoQ couple when there is metabolic oversupply as a reduced CoQ couple allows reverse electron flow through complex I and insulin resistance.
Insulin resistance, via reverse electron flow through complex I, is what is wanted, heat is a by-product.
Insulin, which activates a long chain fatty acid ligase to generate acyl-CoAs for anabolism/triglyceride formation, might well be expected to simultaneously generate the glycerol 3 phosphate needed for the triglyceride backbone. The diversion of FFAs to intracellular triglycerides can be viewed as protection against excess metabolic substrate supply.
It strikes me as appropriate that the same molecule might also be used to generate the insulin resistance which limits substrate oversupply supply, if there is no fat available.
I think I might leave this as a short post before we go on to the post-obese and insulin induced thermogenesis. And possibly on to mtG3Pdh knock out mice.
Peter
Back when talking about the Pima paper I skipped over insulin induced thermogenesis, a fascinating subject and a phenomenon distinctly lacking in the obese, if they are insulin resistant. This is the pattern of IIT, from normal glucose tolerance through to diabetes. From Fig 2:
The phrase "insulin induced thermogenesis" is, at first glance, a complete oxymoron. It is difficult to imagine any way in which insulin, that most effective suppressor of free fatty acids, might uncouple respiration to generate heat. The primary brown adipose tissue technique is to use UCP1, which depends on free fatty acid availability. But there are other ways to generate heat in addition to uncoupling the proton gradient. It is worth noting that some of the thermogenesis is "obligatory", by which the authors mean the exothermic storage of glucose as glycogen. I'm rather more interested in the "facultative" component.
Brand's group have been hard at work again and have this (excellent) paper out:
Sites of reactive oxygen species generation by mitochondria oxidizing different substrates
most especially the lovely figure 1 (use the link to get the legend, it's a good summary of ETC energetics and "non-stressed" superoxide production):
It is an abstraction of the ETC, grouping inputs by their redox potential rather than physical location and is now becoming pretty inclusive for most electron donors. All mitochondrial NADH inputs are through complex I at -280mV and these electrons flow from here towards the positive potential of +600mV, provided by oxygen at complex IV. They do work in the process, pumping protons to set up the delta psi which can be "wasted" to generate heat when needed. Without uncoupling, the process is efficient and energy from the electron is largely conserved.
The interesting point is that mtG3Pdh inputs to the CoQ couple which has, we can now see, a redox potential of +20mV.
Specifically, mtG3Pdh is taking a cytoplasmic NADH, which could theoretically be shuttled to the mitochondrial matrix, and hence to complex I, at -280mV, and inputting it to the ETC at +20mV. The energy lost by skipping from -280mV to +20mV would normally pump four protons and now appears as heat.
After a glucose load any oxidation of the abnormally elevated FFAs of insulin resistant people still provides a continuous +20mV input using ETF dehydrogenase's FADH2 acting on the CoQ couple, which is ultimately derived from the FADH2 of the first step of beta oxidation, ie it's FADH2 transporting electrons all the way, there is no energy wastage when using fats to limit insulin's action. In the absence of these inappropriate FFAs, the correct way to reduce the CoQ couple is using mtG3Pdh which shuts down insulin's action at the cost of generating heat because it uses NADH, stepped down to FADH2, as a direct input at the CoQ level. We want a reduced the CoQ couple when there is metabolic oversupply as a reduced CoQ couple allows reverse electron flow through complex I and insulin resistance.
Insulin resistance, via reverse electron flow through complex I, is what is wanted, heat is a by-product.
Insulin, which activates a long chain fatty acid ligase to generate acyl-CoAs for anabolism/triglyceride formation, might well be expected to simultaneously generate the glycerol 3 phosphate needed for the triglyceride backbone. The diversion of FFAs to intracellular triglycerides can be viewed as protection against excess metabolic substrate supply.
It strikes me as appropriate that the same molecule might also be used to generate the insulin resistance which limits substrate oversupply supply, if there is no fat available.
I think I might leave this as a short post before we go on to the post-obese and insulin induced thermogenesis. And possibly on to mtG3Pdh knock out mice.
Peter
Wednesday, November 27, 2013
Protons (30) Uncoupling and metabolic rate in insulin resistance
I wanted to look at insulin resistance, uncoupling and metabolic rate. If we just review the effect of an intravenous bolus of palmitic acid on an anaesthetised rat we can see that the bolus produces a period of increased oxygen consumption, ie an increased metabolic rate, in a then-uncoupled healthy rat. From Curi again:
This next graphic is looking at glucose oxidation, rather than oxygen consumption, from the same paper.
The left hand graph shows what happens under basal metabolism, ie glucose is being taken up by isolated muscle cells without help from insulin facilitated GLUT4 translocation. Fatty acids uncouple, ATP levels fall, there is an increase in glucose oxidation to compensate, ATP levels are corrected. Presumably some of the fatty acids get metabolised too.
On the right is the effect under supra maximal insulin. We have no idea of dose response curve to insulin from this study, it describes either using none or using 10mU/ml. That's mU, not microU! At a concentration of 10mU/ml insulin will overcome any suggestion of insulin resistance, short of a knockout model. Insulin supports glucose oxidation, uncoupling increases this. The set up is not designed to look at insulin resistance as might be related to that uncoupling. But the effects on overall metabolic rate, at 5.6mmol of glucose, are quite clear cut.
What if that elevated FFA level was maintained long term?
As adipocytes become progressively more resistant to the the anti-lipolytic effect of insulin (why is another whole ball game), plasma free fatty acids rise even under levels of insulin which should be suppressing them. Unless these free fatty acids are converted to CoA derivatives they are will uncouple respiration. This should reduce delta psi and increase metabolic rate.
A reduced delta psi will not support reverse electron flow through complex I. The essential insulin induced pulse of superoxide, converted to H2O2, will not occur. There will be fasting insulin resistance. This paper spelled it out. In brief:
The insulin signalling cascade is tonically restrained by a phosphatase which deactivates the insulin/receptor complex (which activates itself by auto-phosphorylation) as soon as the process tries to get started. For insulin to signal you need to have an elevated delta psi which allows a nanomolar pulse of H2O2 to cripple this phosphatase and so allow the insulin/receptor complex to get signalling.
With reduced delta psi this isn't going to happen. We have enhanced insulin resistance of starvation until the time when food arrives.
You can increase delta psi, of course, to get insulin working. Increasing delta psi requires increased electrons in to the respiratory chain through complex I. In the blunted insulin signalling situation of low delta psi we can't use GLUT4 transporters but we can, given high enough plasma glucose levels, get sufficient glucose in to the cell to generate enough of a delta psi to allow the pulse of H2O2 and its downstream effects to occur. So overcome the inability of insulin to signal in the presence of FFAs.
The cost is that an elevated blood glucose level is needed. This is what we look at with the ratio of fasting glucose to fasting insulin, the HOMA score. An elevated HOMA score is a marker of UNCOUPLING at the delta psi level in mitochondria. It should be associated with an increased metabolic rate in proportion to the degree of fatty acid induced uncoupling.
Does this happen in real life? These data are from the Pima but the pattern is generic in insulin resistant states:
Note first that the changes in FFA concentrations are statistically non significant, but that the trend is nicely upwards with insulin levels
The authors comment:
"Alternatively, FFA may contribute to increased RMR via stimulation of mitochondrial uncoupling proteins (UCPs) (53,54). As early as 1976, Himms-Hagen (53) suggested that FFAs stimulate UCP-1. More recently, elevated FFA concentrations were reported to stimulate UCP-3 expression in rats (54)"
Bear in mind that as people progress from NGT through IGT to diabetes there are not only progressive changes in blood lipid and glucose levels but also progressive damage to mitochondria per se, which makes comparing a healthy rat to a metabolically challenged human being slightly dubious.
But the changes make sense.
If we look at the post prandial situation we have, after a carbohydrate containing meal, the combination of chronically elevated FFAs with acutely elevated glucose. There will be a high delta psi as soon as blood glucose rises high enough (supra physiological) to allow non-GLUT4 uptake to elevate delta psi high enough for insulin signalling. At this time point the mitochondria allow insulin to function. We can now translocate GLUT4s to the cell surface and start to lower postprandial hyperglycaemia by pouring metabolites through glycolysis or in to glycogen stores.
At the same time, among insulin's many diverse functions, the activation of free fatty acids to their CoA form increases, with a view to anabolic or storage functions. I would presume a different CoA ligase directs the activated fatty acids to oxidation. There is quite a group of LCFA CoA ligases.
At this point we lose the freedom of free fatty acids, they are ligated to CoA and suddenly become effective inhibitors of uncoupling rather than facilitators. We are now set up for the post prandial state. As soon as we have access to glycolysis combined with beta oxidation we have the possibility to generate marked reverse electron flow through complex I and re-inhibit the action of insulin using much greater generation of H2O2 than is needed for insulin's initial activation.
Metabolically, the mitochondria do this at this time to stop caloric overload in any individual cell, by diverting excess calories to storage in adipocytes. From the NADH:FADH2 ratio, long chain saturated fats do this best. Monounsaturates appear to be designed to allow a normal combination of glucose and fatty acid oxidation and PUFA fail to generate adequate insulin resistance to protect an individual cell (including adipocytes) from an overload of metabolic substrate. In the immediate post prandial state saturated fats can best protect cells from an absorptive excess of metabolites. These fatty acids are already present in supraphysiological levels, whatever has been eaten.
The elevated insulin needed to maintain post prandial normoglycaemia will, if the adipocytes are able to respond at all, divert fat from the circulation and into those adipocytes. This is the simple ability of insulin, and cellular resistance to insulin, to limit metabolic injury when calories are present in excess of immediate needs.
*****
Under inappropriately elevated FFAs there is an initial failure of insulin's action due to depressed delta psi during fasting and a subsequent post-meal failure of insulin's action due to inappropriately elevated fatty acid metabolism through electron transport flavoprotein dehydrogenase's FADH2 when combined with high delta psi.
To me, this looks very much like impaired metabolic flexibility, reduced to the level of delta psi and superoxide. I like it.
Peter
This next graphic is looking at glucose oxidation, rather than oxygen consumption, from the same paper.
The left hand graph shows what happens under basal metabolism, ie glucose is being taken up by isolated muscle cells without help from insulin facilitated GLUT4 translocation. Fatty acids uncouple, ATP levels fall, there is an increase in glucose oxidation to compensate, ATP levels are corrected. Presumably some of the fatty acids get metabolised too.
On the right is the effect under supra maximal insulin. We have no idea of dose response curve to insulin from this study, it describes either using none or using 10mU/ml. That's mU, not microU! At a concentration of 10mU/ml insulin will overcome any suggestion of insulin resistance, short of a knockout model. Insulin supports glucose oxidation, uncoupling increases this. The set up is not designed to look at insulin resistance as might be related to that uncoupling. But the effects on overall metabolic rate, at 5.6mmol of glucose, are quite clear cut.
What if that elevated FFA level was maintained long term?
As adipocytes become progressively more resistant to the the anti-lipolytic effect of insulin (why is another whole ball game), plasma free fatty acids rise even under levels of insulin which should be suppressing them. Unless these free fatty acids are converted to CoA derivatives they are will uncouple respiration. This should reduce delta psi and increase metabolic rate.
A reduced delta psi will not support reverse electron flow through complex I. The essential insulin induced pulse of superoxide, converted to H2O2, will not occur. There will be fasting insulin resistance. This paper spelled it out. In brief:
The insulin signalling cascade is tonically restrained by a phosphatase which deactivates the insulin/receptor complex (which activates itself by auto-phosphorylation) as soon as the process tries to get started. For insulin to signal you need to have an elevated delta psi which allows a nanomolar pulse of H2O2 to cripple this phosphatase and so allow the insulin/receptor complex to get signalling.
With reduced delta psi this isn't going to happen. We have enhanced insulin resistance of starvation until the time when food arrives.
You can increase delta psi, of course, to get insulin working. Increasing delta psi requires increased electrons in to the respiratory chain through complex I. In the blunted insulin signalling situation of low delta psi we can't use GLUT4 transporters but we can, given high enough plasma glucose levels, get sufficient glucose in to the cell to generate enough of a delta psi to allow the pulse of H2O2 and its downstream effects to occur. So overcome the inability of insulin to signal in the presence of FFAs.
The cost is that an elevated blood glucose level is needed. This is what we look at with the ratio of fasting glucose to fasting insulin, the HOMA score. An elevated HOMA score is a marker of UNCOUPLING at the delta psi level in mitochondria. It should be associated with an increased metabolic rate in proportion to the degree of fatty acid induced uncoupling.
Does this happen in real life? These data are from the Pima but the pattern is generic in insulin resistant states:
Note first that the changes in FFA concentrations are statistically non significant, but that the trend is nicely upwards with insulin levels
The authors comment:
"Alternatively, FFA may contribute to increased RMR via stimulation of mitochondrial uncoupling proteins (UCPs) (53,54). As early as 1976, Himms-Hagen (53) suggested that FFAs stimulate UCP-1. More recently, elevated FFA concentrations were reported to stimulate UCP-3 expression in rats (54)"
Bear in mind that as people progress from NGT through IGT to diabetes there are not only progressive changes in blood lipid and glucose levels but also progressive damage to mitochondria per se, which makes comparing a healthy rat to a metabolically challenged human being slightly dubious.
But the changes make sense.
If we look at the post prandial situation we have, after a carbohydrate containing meal, the combination of chronically elevated FFAs with acutely elevated glucose. There will be a high delta psi as soon as blood glucose rises high enough (supra physiological) to allow non-GLUT4 uptake to elevate delta psi high enough for insulin signalling. At this time point the mitochondria allow insulin to function. We can now translocate GLUT4s to the cell surface and start to lower postprandial hyperglycaemia by pouring metabolites through glycolysis or in to glycogen stores.
At the same time, among insulin's many diverse functions, the activation of free fatty acids to their CoA form increases, with a view to anabolic or storage functions. I would presume a different CoA ligase directs the activated fatty acids to oxidation. There is quite a group of LCFA CoA ligases.
At this point we lose the freedom of free fatty acids, they are ligated to CoA and suddenly become effective inhibitors of uncoupling rather than facilitators. We are now set up for the post prandial state. As soon as we have access to glycolysis combined with beta oxidation we have the possibility to generate marked reverse electron flow through complex I and re-inhibit the action of insulin using much greater generation of H2O2 than is needed for insulin's initial activation.
Metabolically, the mitochondria do this at this time to stop caloric overload in any individual cell, by diverting excess calories to storage in adipocytes. From the NADH:FADH2 ratio, long chain saturated fats do this best. Monounsaturates appear to be designed to allow a normal combination of glucose and fatty acid oxidation and PUFA fail to generate adequate insulin resistance to protect an individual cell (including adipocytes) from an overload of metabolic substrate. In the immediate post prandial state saturated fats can best protect cells from an absorptive excess of metabolites. These fatty acids are already present in supraphysiological levels, whatever has been eaten.
The elevated insulin needed to maintain post prandial normoglycaemia will, if the adipocytes are able to respond at all, divert fat from the circulation and into those adipocytes. This is the simple ability of insulin, and cellular resistance to insulin, to limit metabolic injury when calories are present in excess of immediate needs.
*****
Under inappropriately elevated FFAs there is an initial failure of insulin's action due to depressed delta psi during fasting and a subsequent post-meal failure of insulin's action due to inappropriately elevated fatty acid metabolism through electron transport flavoprotein dehydrogenase's FADH2 when combined with high delta psi.
To me, this looks very much like impaired metabolic flexibility, reduced to the level of delta psi and superoxide. I like it.
Peter
Sunday, November 24, 2013
Death by dogma
It's amazing what you can find on the internet when you click on a link. I stumbled over this recently:
Premature aging in mice activates a systemic metabolic response involving autophagy induction
I picked up this gem of a paper from, of all places, a blog with somewhat limited enthusiasm for ketogenic eating. I'll just go through the results section of the paper, giving a staccato summary of each paragraph, because you have to be sure of exactly what a group have found before you consider whether you agree with their conclusions. These mice lack the ability to form prelamin A correctly, instead they form progerin, and they age very rapidly. Here we go with the results section:
A mutation which damages nuclear architecture and causes premature aging also increases autophagy.
The abnormal protein formed (that prelamin A precursor known as progerin) appears to be the cause of premature aging and to be associated with increased autophagy (in this model).
Other models, XPF and CSB/XPA, of rapidly aging mice (both with defective DNA repair processes) do the same thing but without accumulating progerin, especially they increase basal autophagy. So this upregulated autophagy is common to several models of premature ageing, not just the prelamin A model.
mTOR signalling is switched off. Really switched off.
The PI3K-Akt pathway, which usually activates mTOR, is not the explanation.
AMPK is switched on. Really switched on.
Stopping the response to DNA damage (p53 knockout) does not stop enhanced AMKP activity. So we are not looking at extra autophagy to recycle damaged DNA.
Next we get on to glucose. The five hour starved level of glucose is low, around 38% of control value. Insulin is low too.
In the liver things are strange.
Phosphoenolpyruvate carboxykinase and glucose-6-phosphatase are up-regulated, both are important for gluconeogenesis.
Puryvate kinase (a glycolysis regulator) is not up-regulated. So where is the glucose going if it's not going to glycolysis?
Glucose from gluconeogenesis appears to end up in the liver as glycogen granules, without needing glycogen synthase to be up regulated. This glycogen can be accessed if needed.
At the same time fatty acid producing genes are up regulated. Glucose is being converted to fatty acids. Genes associated with fatty acid oxidation are up regulated too. And a fatty liver develops. Very interesting.
Pyruvate dehydrogenase kinase-4, key for switching from glucose to fat burning, is strikingly upregulated. These mice burn fat. They reject glucose. And they die of precocious aging!
All of the "good" markers indicating longevity in many models are fantastic in these mice. The end product is early death.
Metabolically, everything appears to come down to PGC 1-alpha. It's production is very upregulated. This cofactor appears to responsible for the switch from glucose to fat based metabolism.
End of results summary.
This is where the paper stops.
On the basis of these findings a concern expressed in the discussion is that elevated PGC 1-alpha drives autophagy, which is initially adaptive but might become maladaptive when chronically activated. This is a potentially valid concern (there is an autophagy triggered form of cell death distinct from apoptosis and necrosis) but we have to bear in mind that there is zero data to support this specific concern provided in the paper. That's all of the results section summarised up above.
The authors are well aware that the reason for rapid aging is the genetic defect in nuclear architecture formation. This leads, indirectly, to genomic instability which immediately puts this model in to the same category as other premature aging models such as XPF and CSB/XPA, both of which have defects in DNA repair, also as mentioned above.
So why do the cells of these animals go in to a state of AMPK driven, mTOR inhibition dependent, persistent autophagy?
They do this because they have a severe ATP deficit. High levels of AMP per unit ATP drive AMPK.
Why is there insufficient ATP?
Let's have a read at Nick Lane's essay Mitonuclear match: optimizing fitness and fertility over generations drives aging within generations. Here's the quote:
"Oxidative phosphorylation involves the transfer of electrons through a succession of redox centres in respiratory chains, from an electron donor such as NADH, to a terminal acceptor such as oxygen. A slowing of electron transfer means that respiratory complexes become more highly reduced, which increases their reactivity with oxygen, corresponding to a rise in free-radical leak [25]. A slowing of electron transfer is the most likely outcome of any mismatch between mitochondrial and nuclear genomes. The reason relates to the mechanism of electron transfer. If the gap between adjacent redox centres in respiratory chains is increased by just 1 A, electron transfer by quantum tunnelling slows down by an order of magnitude [26] and free-radical leak should rise accordingly. Given that hydrogen bonds and Van der Waal’s forces act over distances in the range of 1–2 A, it is likely that any changes to optimal subunit interactions would disrupt the distance between redox centres by more than 1 A, slowing electron flow and increasing free-radical leak. Thus, a rise in free-radical leak is the predicted outcome of virtually any subunit mismatch, and indeed has been reported [27, 28]"
Question: How well might the electron transport chain function in the mitochondria of a cell which has a permanent defect in its ability to repair nuclear DNA damage?
Answer: Three separate mouse models, engineered to have defective DNA repair, have chronically activated AMPK.
I think these mice are "starving" with full stomachs because they cannot generate ATP. They have progressive un-repaired changes to the nuclear DNA coding for (amongst many things) electron transport chain components which means these proteins simply don't fit the proteins from mtDNA.
Soooooooo. Essentially all of the changes in these mice could be traced back to inadequate ATP generation, with the added bonus of excess ROS generation to further damage the "unrepairable" DNA.
The extended autophagy is a total red herring, sort of.
I picked this paper up as a FB link "liked" by Chris Highcock. The linked blogger is so impressed by the lethal effect of autophagy that he appears to consider that diet-induced autophagy is a "worthless dogma" and avoids it, not liking dogma. That's fine by me, we all have our quirks.
Unfortunately for this autophago-phobic concept, and possibly for the poor chap himself, it turns out that if you take a prelamin A mutant mouse and treat it with rapamycin you will actually promote even MORE autophagy. So, if autophagy is the cause of premature aging, things should get worse. Yes? The actual result is that:
"Here, we report the discovery of rapamycin as a novel inhibitor of progerin [defective prelamin A], which dramatically and selectively decreases protein levels through a mechanism involving autophagic degradation. Rapamycin treatment of progeria cells lowers progerin, as well as wild-type prelamin A levels, and rescues the chromatin phenotype of cultured fibroblasts..."
or just have the title:
Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria.
As an approach, it works. Further increasing autophagy rescues prelamin A mutant mouse cells. RIP autophagy as a "cause" of accelerated aging.
I like Chris a lot, he's a really nice chap. Luckily the sort of hill walking he does (still love your Pentland pictures on FB, if you read this Chris) he will be regularly and repeatedly activating AMPK with subsequently increased autophagy. Exercise does this. So does the "misery" of ketogenic eating.
Actively changing your diet to avoid autophagy, based on a progerin model which can actually be rescued by increasing autophagy, just might be a booboo. Imitate with caution.
Peter
BTW It's sort of nice looking at technical papers which (superficially) challenge my low carbohydrate biased perspective. Knowing you are in a correct paradigm makes dissecting them a rather relaxed process when you start form a position where the world makes sense. Perhaps this is dogma.
Premature aging in mice activates a systemic metabolic response involving autophagy induction
I picked up this gem of a paper from, of all places, a blog with somewhat limited enthusiasm for ketogenic eating. I'll just go through the results section of the paper, giving a staccato summary of each paragraph, because you have to be sure of exactly what a group have found before you consider whether you agree with their conclusions. These mice lack the ability to form prelamin A correctly, instead they form progerin, and they age very rapidly. Here we go with the results section:
A mutation which damages nuclear architecture and causes premature aging also increases autophagy.
The abnormal protein formed (that prelamin A precursor known as progerin) appears to be the cause of premature aging and to be associated with increased autophagy (in this model).
Other models, XPF and CSB/XPA, of rapidly aging mice (both with defective DNA repair processes) do the same thing but without accumulating progerin, especially they increase basal autophagy. So this upregulated autophagy is common to several models of premature ageing, not just the prelamin A model.
mTOR signalling is switched off. Really switched off.
The PI3K-Akt pathway, which usually activates mTOR, is not the explanation.
AMPK is switched on. Really switched on.
Stopping the response to DNA damage (p53 knockout) does not stop enhanced AMKP activity. So we are not looking at extra autophagy to recycle damaged DNA.
Next we get on to glucose. The five hour starved level of glucose is low, around 38% of control value. Insulin is low too.
In the liver things are strange.
Phosphoenolpyruvate carboxykinase and glucose-6-phosphatase are up-regulated, both are important for gluconeogenesis.
Puryvate kinase (a glycolysis regulator) is not up-regulated. So where is the glucose going if it's not going to glycolysis?
Glucose from gluconeogenesis appears to end up in the liver as glycogen granules, without needing glycogen synthase to be up regulated. This glycogen can be accessed if needed.
At the same time fatty acid producing genes are up regulated. Glucose is being converted to fatty acids. Genes associated with fatty acid oxidation are up regulated too. And a fatty liver develops. Very interesting.
Pyruvate dehydrogenase kinase-4, key for switching from glucose to fat burning, is strikingly upregulated. These mice burn fat. They reject glucose. And they die of precocious aging!
All of the "good" markers indicating longevity in many models are fantastic in these mice. The end product is early death.
Metabolically, everything appears to come down to PGC 1-alpha. It's production is very upregulated. This cofactor appears to responsible for the switch from glucose to fat based metabolism.
End of results summary.
This is where the paper stops.
On the basis of these findings a concern expressed in the discussion is that elevated PGC 1-alpha drives autophagy, which is initially adaptive but might become maladaptive when chronically activated. This is a potentially valid concern (there is an autophagy triggered form of cell death distinct from apoptosis and necrosis) but we have to bear in mind that there is zero data to support this specific concern provided in the paper. That's all of the results section summarised up above.
The authors are well aware that the reason for rapid aging is the genetic defect in nuclear architecture formation. This leads, indirectly, to genomic instability which immediately puts this model in to the same category as other premature aging models such as XPF and CSB/XPA, both of which have defects in DNA repair, also as mentioned above.
So why do the cells of these animals go in to a state of AMPK driven, mTOR inhibition dependent, persistent autophagy?
They do this because they have a severe ATP deficit. High levels of AMP per unit ATP drive AMPK.
Why is there insufficient ATP?
Let's have a read at Nick Lane's essay Mitonuclear match: optimizing fitness and fertility over generations drives aging within generations. Here's the quote:
"Oxidative phosphorylation involves the transfer of electrons through a succession of redox centres in respiratory chains, from an electron donor such as NADH, to a terminal acceptor such as oxygen. A slowing of electron transfer means that respiratory complexes become more highly reduced, which increases their reactivity with oxygen, corresponding to a rise in free-radical leak [25]. A slowing of electron transfer is the most likely outcome of any mismatch between mitochondrial and nuclear genomes. The reason relates to the mechanism of electron transfer. If the gap between adjacent redox centres in respiratory chains is increased by just 1 A, electron transfer by quantum tunnelling slows down by an order of magnitude [26] and free-radical leak should rise accordingly. Given that hydrogen bonds and Van der Waal’s forces act over distances in the range of 1–2 A, it is likely that any changes to optimal subunit interactions would disrupt the distance between redox centres by more than 1 A, slowing electron flow and increasing free-radical leak. Thus, a rise in free-radical leak is the predicted outcome of virtually any subunit mismatch, and indeed has been reported [27, 28]"
Question: How well might the electron transport chain function in the mitochondria of a cell which has a permanent defect in its ability to repair nuclear DNA damage?
Answer: Three separate mouse models, engineered to have defective DNA repair, have chronically activated AMPK.
I think these mice are "starving" with full stomachs because they cannot generate ATP. They have progressive un-repaired changes to the nuclear DNA coding for (amongst many things) electron transport chain components which means these proteins simply don't fit the proteins from mtDNA.
Soooooooo. Essentially all of the changes in these mice could be traced back to inadequate ATP generation, with the added bonus of excess ROS generation to further damage the "unrepairable" DNA.
The extended autophagy is a total red herring, sort of.
I picked this paper up as a FB link "liked" by Chris Highcock. The linked blogger is so impressed by the lethal effect of autophagy that he appears to consider that diet-induced autophagy is a "worthless dogma" and avoids it, not liking dogma. That's fine by me, we all have our quirks.
Unfortunately for this autophago-phobic concept, and possibly for the poor chap himself, it turns out that if you take a prelamin A mutant mouse and treat it with rapamycin you will actually promote even MORE autophagy. So, if autophagy is the cause of premature aging, things should get worse. Yes? The actual result is that:
"Here, we report the discovery of rapamycin as a novel inhibitor of progerin [defective prelamin A], which dramatically and selectively decreases protein levels through a mechanism involving autophagic degradation. Rapamycin treatment of progeria cells lowers progerin, as well as wild-type prelamin A levels, and rescues the chromatin phenotype of cultured fibroblasts..."
or just have the title:
Autophagic degradation of farnesylated prelamin A as a therapeutic approach to lamin-linked progeria.
As an approach, it works. Further increasing autophagy rescues prelamin A mutant mouse cells. RIP autophagy as a "cause" of accelerated aging.
I like Chris a lot, he's a really nice chap. Luckily the sort of hill walking he does (still love your Pentland pictures on FB, if you read this Chris) he will be regularly and repeatedly activating AMPK with subsequently increased autophagy. Exercise does this. So does the "misery" of ketogenic eating.
Actively changing your diet to avoid autophagy, based on a progerin model which can actually be rescued by increasing autophagy, just might be a booboo. Imitate with caution.
Peter
BTW It's sort of nice looking at technical papers which (superficially) challenge my low carbohydrate biased perspective. Knowing you are in a correct paradigm makes dissecting them a rather relaxed process when you start form a position where the world makes sense. Perhaps this is dogma.
Thursday, November 14, 2013
Ketoacidotic death in lactation!
Laura forwarded me the link to this epic study:
A gestational ketogenic diet alters maternal metabolic status as well as offspring physiological growth and brain structure in the neonatal mouse.
It has a very very clear message. This is the first paragraph of the results section:
"Lactation in KD [ketogenic diet fed] Dams Leads to Fatal Ketoacidosis. In the initial trial phase, 4 KD dams remained with their biological litters post parturition, and during lactation. However, lactation appeared to cause severe physiological strain to all KD dams. Soon after parturition they started exhibiting signs of high stress, as was also apparent by them cannibalizing their litter. Measurements of their blood glucose and ketone revealed elevated values (Glucose: ∼20 mmol/L and Ketone: ∼6 mmol/L), indicating development of ketoacidosis. This ketoacidosis rapidly progressed to a fatal metabolic state, leading to the death of the KD dams within a few days post parturition"
I hope that is clear. No beating about the bush.
These folks are cutting edge. They know how to get impressive results.
Take home message: If you wish to survive lactation do not, UNDER ANY CIRCUMSTANCES, base your diet on CRISCO. Do not do it.
My wife seems to have survived two ketogenic lactations in pretty good shape. Daniel's school is well in to spelling test this term. He is the only kid in his class to have made no mistakes so far. It doesn't look as if we have broken his brain. No Crisco.
Of course Sussman et al blame the ketosis. They keep very quiet about the Crisco:
TD.96355 Ketogenic Diet: "Vegetable Shortening, hydrogenated (Crisco) 586.4g/kg"
Want to die? Always ask for Crisco by name. Crooks.
Peter
A gestational ketogenic diet alters maternal metabolic status as well as offspring physiological growth and brain structure in the neonatal mouse.
It has a very very clear message. This is the first paragraph of the results section:
"Lactation in KD [ketogenic diet fed] Dams Leads to Fatal Ketoacidosis. In the initial trial phase, 4 KD dams remained with their biological litters post parturition, and during lactation. However, lactation appeared to cause severe physiological strain to all KD dams. Soon after parturition they started exhibiting signs of high stress, as was also apparent by them cannibalizing their litter. Measurements of their blood glucose and ketone revealed elevated values (Glucose: ∼20 mmol/L and Ketone: ∼6 mmol/L), indicating development of ketoacidosis. This ketoacidosis rapidly progressed to a fatal metabolic state, leading to the death of the KD dams within a few days post parturition"
I hope that is clear. No beating about the bush.
These folks are cutting edge. They know how to get impressive results.
Take home message: If you wish to survive lactation do not, UNDER ANY CIRCUMSTANCES, base your diet on CRISCO. Do not do it.
My wife seems to have survived two ketogenic lactations in pretty good shape. Daniel's school is well in to spelling test this term. He is the only kid in his class to have made no mistakes so far. It doesn't look as if we have broken his brain. No Crisco.
Of course Sussman et al blame the ketosis. They keep very quiet about the Crisco:
TD.96355 Ketogenic Diet: "Vegetable Shortening, hydrogenated (Crisco) 586.4g/kg"
Want to die? Always ask for Crisco by name. Crooks.
Peter
Labels:
Ketoacidotic death in lactation!
Tuesday, November 12, 2013
Protons (29) Uncoupling with fatty acids
Trying to work out exactly what is happening in a given functional component of the inner mitochondrial membrane is not the most simple of undertakings. For anyone who wishes to bend their brain a little you could do worse than working through Federenko's paper on the likely structure/function of UCP1 in brown adipose tissue. I think the original pdf was sent to me by Alex, some time ago. Although it is looking at UCP1 I think we can reasonably assume other UCPs work in much the same manner. This set of line doodles summarises the paper:
Here's the explanatory text:
(A) The simplest mechanism of steady H+ IUCP1 induced by LCFAs. UCP1 operates as a symporter that transports one LCFA and one H+ per the transport cycle. First, the LCFA anion binds to UCP1 on the cytosolic side at the bottom of a hypothetical cavity (1). H+ binding to UCP1 occurs only after the LCFA anion binds to UCP1 (1). The H+ and the LCFA are translocated by UCP1 upon conformational change, and H+ is released on the opposite side of the IMM, whereas the LCFA anion stays associated with UCP1 due to the hydrophobic interactions established by its carbon tail (2). The LCFA anion then returns to initiate another H+ translocation cycle (3). Charge is translocated only in step 3 when the LCFA anion returns without the H+.
All nice and simple, compared to how complex it was to work all of this out. In plain English: The (removable) fatty acid flip-flops back and forth, passing a proton each time. UCPs absolutely MUST have access to long chain fatty acids to function. The LCFAs must be free, ie NOT be activated. Sticking a large CoA moiety on to the red dot (which is the carboxylic end of the LCFA) will completely inhibit any activity in UCP1 as there is then no negatively charged binding site on the fatty acid to allow it to ferry protons, repeatedly, down their concentration gradient back in to the mitochondrial matrix.
So fatty acids may just be slightly important to uncoupling. Uncoupling may be a Good Thing or the converse, depending on your point of view or your carbohydrate intake.
This leads us on to Curi's group and their 2006 attempt to look for the effect of FFAs on uncoupling in tissue culture, in isolated mitochondria and even in whole live rats. When you see a line in the methods which specifically states "in Krebs–Ringer bicarbonate buffer containing 5.6 mM glucose" you know you are in for a treat. You have to have read as much cell culture literature as I have to realise how precious a line like this is. All you normally get is the type of medium, probably the brand name and the supplier, but oops, forgot to mention it has, or might have, or might not have, 25mmol of glucose in it. Or some other random amount. Duh. The easy way to tell (when not specified) is that if saturated fats cause apoptosis in cell culture, be certain that glucose is >20mmol.
Anyhoo. Here we go:
This is from first generation harvested and cultured skeletal muscle cells. The fluorescence rises with inner mitochondrial membrane potential, delta psi. Antimycin A blocks ATPase so hyperpolarises the membrane, CCCP is a chemical uncoupler and so reduces it. These are just tests to show the prep works. The caprylic acid is useless (it works well on isolate mitochondria but the cytoplasm probably stops MCTs reaching the mitochondria without adding CoA while they are on their way there). Palmitic acid and linoleic acid uncouple, like a mild version of CCCP.
You can show exactly the same effect in chronically cultured myotubules, just another cellular prep.
This is our first look at isolated mitochondria. This is a graph of the same delta psi sensitive fluorescent dye plotted against time. The graph is upside down because, in real life, delta psi is negative. You add SMM (skeletal muscle mitochondria) to the observation medium. They fire up and delta psi increases. This is the curve dropping downwards from the SMM arrow. In the middle of the 2 minute bar, palmitic acid is added and delta psi decreases (upward flick in the upside down graph) marked by the PA arrow. The bottom line is what happens if you have added guanidine di phosphate, GDP, an inhibitor of UCPs. At the end they also added CCCP to fully uncouple the mitochondrial membrane and show that delta psi collapses.
Note, you can only show the inhibitory effect of GDP when the CoQ couple is oxidised. A reduced CoQ couple stops GDP's inhibitory effect on uncoupling. This gives a mass of contradictory studies in the literature, needless to say. The control of uncoupling as a fascinating area, perhaps another post.
And this graph is looking at the rise in oxygen consumption induced by uncoupling using assorted fatty acids. Again, mitochondria, so caprylic acid works, though never as well as the longer fatty acids. Note the lack of GDP effect, the CoQ couple must be reduced in this prep. BSA (bovine serum albumin) eliminates the uncoupling as it "hoovers-up" all available FFAs.
Does this uncoupling happen in intact animals? Yes. A stack of caveats, but yes. Ratties were bolused with intravenous palmitate or alcohol (As the control??!!?? Alcohol is not inert, even if it doesn't uncouple) and then oxygen consumption was measured:
Palmitic acid uncouples.
Right, that will do for now. The plan is to look at FFAs and insulin resistance next, within this framework.
Peter, chronically uncoupled.
Here's the explanatory text:
(A) The simplest mechanism of steady H+ IUCP1 induced by LCFAs. UCP1 operates as a symporter that transports one LCFA and one H+ per the transport cycle. First, the LCFA anion binds to UCP1 on the cytosolic side at the bottom of a hypothetical cavity (1). H+ binding to UCP1 occurs only after the LCFA anion binds to UCP1 (1). The H+ and the LCFA are translocated by UCP1 upon conformational change, and H+ is released on the opposite side of the IMM, whereas the LCFA anion stays associated with UCP1 due to the hydrophobic interactions established by its carbon tail (2). The LCFA anion then returns to initiate another H+ translocation cycle (3). Charge is translocated only in step 3 when the LCFA anion returns without the H+.
All nice and simple, compared to how complex it was to work all of this out. In plain English: The (removable) fatty acid flip-flops back and forth, passing a proton each time. UCPs absolutely MUST have access to long chain fatty acids to function. The LCFAs must be free, ie NOT be activated. Sticking a large CoA moiety on to the red dot (which is the carboxylic end of the LCFA) will completely inhibit any activity in UCP1 as there is then no negatively charged binding site on the fatty acid to allow it to ferry protons, repeatedly, down their concentration gradient back in to the mitochondrial matrix.
So fatty acids may just be slightly important to uncoupling. Uncoupling may be a Good Thing or the converse, depending on your point of view or your carbohydrate intake.
This leads us on to Curi's group and their 2006 attempt to look for the effect of FFAs on uncoupling in tissue culture, in isolated mitochondria and even in whole live rats. When you see a line in the methods which specifically states "in Krebs–Ringer bicarbonate buffer containing 5.6 mM glucose" you know you are in for a treat. You have to have read as much cell culture literature as I have to realise how precious a line like this is. All you normally get is the type of medium, probably the brand name and the supplier, but oops, forgot to mention it has, or might have, or might not have, 25mmol of glucose in it. Or some other random amount. Duh. The easy way to tell (when not specified) is that if saturated fats cause apoptosis in cell culture, be certain that glucose is >20mmol.
Anyhoo. Here we go:
This is from first generation harvested and cultured skeletal muscle cells. The fluorescence rises with inner mitochondrial membrane potential, delta psi. Antimycin A blocks ATPase so hyperpolarises the membrane, CCCP is a chemical uncoupler and so reduces it. These are just tests to show the prep works. The caprylic acid is useless (it works well on isolate mitochondria but the cytoplasm probably stops MCTs reaching the mitochondria without adding CoA while they are on their way there). Palmitic acid and linoleic acid uncouple, like a mild version of CCCP.
You can show exactly the same effect in chronically cultured myotubules, just another cellular prep.
This is our first look at isolated mitochondria. This is a graph of the same delta psi sensitive fluorescent dye plotted against time. The graph is upside down because, in real life, delta psi is negative. You add SMM (skeletal muscle mitochondria) to the observation medium. They fire up and delta psi increases. This is the curve dropping downwards from the SMM arrow. In the middle of the 2 minute bar, palmitic acid is added and delta psi decreases (upward flick in the upside down graph) marked by the PA arrow. The bottom line is what happens if you have added guanidine di phosphate, GDP, an inhibitor of UCPs. At the end they also added CCCP to fully uncouple the mitochondrial membrane and show that delta psi collapses.
Note, you can only show the inhibitory effect of GDP when the CoQ couple is oxidised. A reduced CoQ couple stops GDP's inhibitory effect on uncoupling. This gives a mass of contradictory studies in the literature, needless to say. The control of uncoupling as a fascinating area, perhaps another post.
And this graph is looking at the rise in oxygen consumption induced by uncoupling using assorted fatty acids. Again, mitochondria, so caprylic acid works, though never as well as the longer fatty acids. Note the lack of GDP effect, the CoQ couple must be reduced in this prep. BSA (bovine serum albumin) eliminates the uncoupling as it "hoovers-up" all available FFAs.
Does this uncoupling happen in intact animals? Yes. A stack of caveats, but yes. Ratties were bolused with intravenous palmitate or alcohol (As the control??!!?? Alcohol is not inert, even if it doesn't uncouple) and then oxygen consumption was measured:
Palmitic acid uncouples.
Right, that will do for now. The plan is to look at FFAs and insulin resistance next, within this framework.
Peter, chronically uncoupled.
Tuesday, October 01, 2013
Polycystic Kidney Disease and mTOR
I think I'm pretty well out of blogging time for the next month or so, so the post looking at fatty acids, uncoupling and insulin resistance will have to sit on the back burner while I work on an anaesthesia project. A friend emailed me a paper with this rather nice diagram of the probable aetiopathology of polycystic kidney disease. Whenever I see mTOR driving pathology I always think of ketogenic eating to obtund the process. If you carry PKD genetics I don't see any other remotely sensible option...
No Reverse Warburg Effect here. I love the 2-deoxyglucose too. If there was ever a way of faking a ketogenic diet, this is it. More so than ketone esters! A bit of physiological insulin resistance or a drug to block glycolysis? Or how about a modern mTOR inhibitor? I'll leave it open to guesswork (or Pubmed if you must) what a ketogenic diet does to mTOR signalling.
Peter
No Reverse Warburg Effect here. I love the 2-deoxyglucose too. If there was ever a way of faking a ketogenic diet, this is it. More so than ketone esters! A bit of physiological insulin resistance or a drug to block glycolysis? Or how about a modern mTOR inhibitor? I'll leave it open to guesswork (or Pubmed if you must) what a ketogenic diet does to mTOR signalling.
Peter
Saturday, September 21, 2013
Electron Transport Chain image
Just off to bed. Wow, do I have wild Saturday nights! Had to share this lovely pic. One of the better representations of the ETC I've ever seen, lifted from here.
Like.
Peter
Like.
Peter
Wooo and the snps
There seems to be quite a bit of interest going on in the LC Hardcore at the moment and I'm sat here, under my stone, looking at UCPs as prime mediators of the insulin resistance of fasting and membrane pumps in the origin of life as relates to lactate and extracellular pH in cancer. And I should really be working on a dead-lined anaesthesia project. Wooo and Toxic ("I read your potatoes, and the news will never be good" LMAO) have both brought up 23andme and, surprise surprise, Wooo has enough snps on assorted ion channel genes to have her in a loony bin several times over. She concludes that a ketogenic diet gets her a normal life.
Obviously I have nothing to disagree with here.
What I would comment on is that a very, very large chunk of the "normal" population may not be quite as normal as she suspects. Having frank bipolar disease with severe enough presentation to give you a clear cut "label" is quite rare. Having severe depression to the point of complete loss of functionality or schizophrenia to the point of obeying the voices completely, whatever they command, are all equally rare. But shades of grey appear to be very common and I don't see that many normal people are all that normal. Undoubtedly we all have snps on all sorts of genes. That's genetic variability and is essential to provide a pool for the species to adapt through.
That many mental illnesses are essentially metabolic, and that ion channels have a great deal to do with neural energy demands, is not exactly unexpected. Sid Dishes emailed me this rather interesting review (BTW finding this in Nature is rather like reading a massive endorsement of the Atkins diet in the Sun or the Daily Mail, Sid feels paradigm shift) looking at exactly the metabolic aspect. Yet another email needing a thankyou not sent yet. Thanks Sid. Look at this quote from the abstract talking about central neurons:
"it is now clear that they [psychiatric illnesses] are associated with impairments of synaptic plasticity"
and tie that back to peripheral neuropathy, here's Chowdhury on peripheral nerves:
"The consequences of suboptimal ATP supply for the distal nerve fiber are numerous: (1) collateral sprouting and plasticity will be retarded, (2) this will lead to gradual pruning of the axonal network and shrinkage of sensory innervation fields, and (3) end organs of myelinated fibers within the dermis will lose innervation and function (see Fig. 3)"
My italics.
The parallels, to me, make it sound like we are talking about the same process. I would suggest that hyperglycaemia breaks the mitochondrial population and ketosis is an excellent sticking plaster. Which snps you have determine which neurons break first.
If you also have metabolic snps which limit your ability to avoid hyperglycaemia on the SAD in addition to neural snps which make for "upper limit of normality" energy demands within hyperglycaemia compromised neurons, you are on your way to the Funny Farm. Or a ketogenic diet.
I, for one, am very glad Wooo hit the ketogenic diet arm.
I have said that I think it is unlikely that humans are in any way adapted to a diet which regularly and severely induces hyperglycaemia. Had Wooo been born in to a normoglycaemic environment, what would the effect have been of her ion channel snps on perception?
If you think Wooo is "normal", you are crazy.
Personally, I think we need people who are three standard deviations from the population norm when it comes to insight and perception. This may well be down to ion channels and snps of the Wooo flavour... I don't see that we would get too far, species wise, if we were all Taterheads with ion channels which allowed tolerance of hyperglycaemia until Alzheimers (type 3 diabetes) kicked in, and yet only allowed as much insight in to anything as a turnip has. Of the latter, there is a lot of it about, we have more than enough.
Blog on Wooo.
Peter
Obviously I have nothing to disagree with here.
What I would comment on is that a very, very large chunk of the "normal" population may not be quite as normal as she suspects. Having frank bipolar disease with severe enough presentation to give you a clear cut "label" is quite rare. Having severe depression to the point of complete loss of functionality or schizophrenia to the point of obeying the voices completely, whatever they command, are all equally rare. But shades of grey appear to be very common and I don't see that many normal people are all that normal. Undoubtedly we all have snps on all sorts of genes. That's genetic variability and is essential to provide a pool for the species to adapt through.
That many mental illnesses are essentially metabolic, and that ion channels have a great deal to do with neural energy demands, is not exactly unexpected. Sid Dishes emailed me this rather interesting review (BTW finding this in Nature is rather like reading a massive endorsement of the Atkins diet in the Sun or the Daily Mail, Sid feels paradigm shift) looking at exactly the metabolic aspect. Yet another email needing a thankyou not sent yet. Thanks Sid. Look at this quote from the abstract talking about central neurons:
"it is now clear that they [psychiatric illnesses] are associated with impairments of synaptic plasticity"
and tie that back to peripheral neuropathy, here's Chowdhury on peripheral nerves:
"The consequences of suboptimal ATP supply for the distal nerve fiber are numerous: (1) collateral sprouting and plasticity will be retarded, (2) this will lead to gradual pruning of the axonal network and shrinkage of sensory innervation fields, and (3) end organs of myelinated fibers within the dermis will lose innervation and function (see Fig. 3)"
My italics.
The parallels, to me, make it sound like we are talking about the same process. I would suggest that hyperglycaemia breaks the mitochondrial population and ketosis is an excellent sticking plaster. Which snps you have determine which neurons break first.
If you also have metabolic snps which limit your ability to avoid hyperglycaemia on the SAD in addition to neural snps which make for "upper limit of normality" energy demands within hyperglycaemia compromised neurons, you are on your way to the Funny Farm. Or a ketogenic diet.
I, for one, am very glad Wooo hit the ketogenic diet arm.
I have said that I think it is unlikely that humans are in any way adapted to a diet which regularly and severely induces hyperglycaemia. Had Wooo been born in to a normoglycaemic environment, what would the effect have been of her ion channel snps on perception?
If you think Wooo is "normal", you are crazy.
Personally, I think we need people who are three standard deviations from the population norm when it comes to insight and perception. This may well be down to ion channels and snps of the Wooo flavour... I don't see that we would get too far, species wise, if we were all Taterheads with ion channels which allowed tolerance of hyperglycaemia until Alzheimers (type 3 diabetes) kicked in, and yet only allowed as much insight in to anything as a turnip has. Of the latter, there is a lot of it about, we have more than enough.
Blog on Wooo.
Peter
Friday, September 06, 2013
Omega 3s and G-protein coupled receptors
Let's just summarise the role of omega 6 fats in Sauer's rat model of cancer:
In the lab situation rapid hepatoma tumour growth needs either arachidonic or linoleic acids. The acids must be taken up in to the hepatoma cells, they must be acted on by lipoxygenase to produce 13-hydroxyoctadecadienoic acid, better known as 13-HODE. 13-HODE appears to be the mitogen which promotes rapid cancer growth. 13-HODE looks like a repair signal gone wrong in cancer cells. Omega 3 fatty acids block omega 6 fatty acid uptake in to hepatoma cells. That's all well and good but the reason I got in to this paper was omega 3 PUFA signalling, rather than those omega 6 issues...
OK, Sauer starts to give some pointers on the function of omega 3 fatty acids in health. That's interesting, as I'm no great lover of any sort of PUFA when I view them from the Protons perspective, yet omega 3s seem to come out pretty well, certainly at low doses. You know my fall back, omega 3 PUFA don't always behave like omega 6 PUFA because they get used as signalling molecules blah blah blah. My own inability to tie the molecular structure of omega 3s to their clinical effects is very frustrating! That they probably act are sites "above" the ETC suggest that they act as what I view as 'high level signals".
Well they do.
The signalling appears to be through a G-protein linked receptor with all of the usual cAMP cascade that follows binding of a ligand to such a receptor. What I found particularly interesting was the effect produced on fat pads of normal rats when EPA (other papers from Sauer suggest all omega 3s act on whichever receptor is involved) was added to the perfusate.
OK, here is a neat little graph taken from here:
This is from fed rats. In the fed state the FFA uptake by the inguinal fat pad of a rat is about 6 mcg/min/gram, white open squares.
Adding EPA at 0.84mmol/l (a bit supraphysiological for EPA but let's let that ride) and FFA uptake by the fat pad drops to zero, or close to zero. Or, in fact, you could argue a suggestion of fatty acid relase, shown as a negative uptake value. Black circles. Fatty acids are not taken up, they end up in the venous effluent in the experiment, plus a little extra.
Whooooah, so do FFAs go through the roof when you take fish oil IRL??
Well no. That's because of this graph from here in the same paper:
Here we have the free fatty acid release from the inguinal fat pad of a healthy rat who has been starved for 48 hours. Fatty acid release is trundling along at about 3mcg/min/gram until EPA is added, again at around 0.8mmol/l. The release of FFAs, in the fasted state, is eliminated. Table 1 in the same paper shows you can get this effect of halting lipolysis in starved rats with under 0.3mmol/l EPA.
Both effects are mediated through a G-protein coupled receptor, ie high level signalling compared to electrons and superoxide in the electron transport chain.
Obviously there are a number of serious problems with this paper but, as a proof of concept, I buy it. I doubt DHA or alpha linolenic acid would work as well (or the group would have used them for this proof of point exercise!) and I think the levels of EPA used produce a very artefactual "switch-like" effect which is probably a graded response. I doubt 0.8mmol/l or even 0.3mmol/l of EPA is exactly physiological but...
Let's suggest that there is a progressive removal of the influence of adipocytes from the FFA flux in/out of plasma as the level of omega 3s in arterial blood increases. Omega 3 fatty acids render adipocytes irrelevant to free fatty acid levels in the plasma.
That is one hell of an idea.
Next we need a brief look at hepatoma cells, again the graph is provided by Sauer and it shows that omega 3 fatty acids, in a G-protein coupled receptor manner, completely turn off the uptake of ALL fatty acids in to hepatoma cells.
If, and it's quite a big "if", the same effects apply to hepatocytes as well as hepatoma cells, we then have a very straightforward mechanism for the protective effects of omega 3 fish oils on hepatic lipidosis. From my point of view this is quite real as there are pretty convincing papers showing that cats, in real life, can be largely protected against the potentially fatal hepatic lipidosis of rapid weight loss by modest doses of omega 3 fatty acids.
Soooo while omega 3s stop the release of all FFAs from adipocytes, they simultaneously stop the uptake of all fatty acids in to the two primary storage organs for fatty acids, adipocytes and liver.
Do plasma FFAs go up or down?
They do, of course, go down. A paradox? Next paper.
Health warning: This paper is so steeped in VLDL and ApoB lipophobia that it makes difficult reading. But there is so little published on FFAs and omega 3 supplementation that it's worth the ondansetron to read it. It's looking at how omega 3 supplements might lower fasting triglycerides, which are the devil incarnate for CVD risk. A huge chunk of VLDL comes from FFAs released from adipocytes and their subsequent repackaging by the liver. Apparently, and I quote from the abstract:
"FO [fish oil] counteracts intracellular lipolysis in adipocytes by suppressing adipose tissue inflammation"
A bit like insulin resistance is caused by "inflammation". Well, maybe it's that simple. They have taken the concept of high level signalling to its 2013 pedestal without looking for basic mechanisms. They have placed the G-protein coupled receptor on to macrophages in the fat pads, which subsequently control the adipocyte lipolysis using cytokines. I haven't checked how good this concept is. Sauer never looked that deeply. Looks a bit modern to me.
Personally I would guess that there are similar receptors on both adipocytes and hepatocytes, but the review does not seem to cover the ability of omega 3s to inhibit general fatty acid uptake by these two tissues. Ah well.
What they do argue is that omega 3 fatty acids upregulate lipoprotein lipase, pretty well whole body. Of course liver and adipocytes ignore this fatty acid bonanza, as above. LPL upregulation is what I needed to know from this paper.
So where do "spare" fatty acids go to? They go to muscles. Upregulated lipoprotein lipase (heralded as the saviour from elevated fasting triglycerides) allows increased lipid release from VLDL to lower those fasting triglycerides. But it's worth bearing in mind that cells do not "see" VLDL, the LPL is on the vascular endothelium and the cells behind the vessel wall only ever receive "free" fatty acids. These are not labelled as from albumin, VLDL or chylomicrons.
Slight aside for later: It seems likely that chylomicrons are going spill their lipids via that same LPL, worth remembering.
The story in the review can be sumarised as omega 3 fatty acids block the release of FFAs from adipocytes and increase the activity of lipoprotein lipase pretty well whole body. VLDL drops, FFAs drop. All is happy in the cardiovascular system. If you believe.
Various "bits" of omega 3s, especially the lipid peroxides of DHA, are signals for mitochondrial biogenesis. I had a paper which specified which lipoxide was most effective but must have missed the "save" button. Mea culpa yet again. There are hints here.
That's a very neat story, which has more than a grain of truth to it.
Why is it like this? What does it mean, physiologcally? Speculation time:
Omega 3 fats come from plants. Mostly from chloroplasts. Where do humans get their omega 3s from? Certainly not from plants. If we did then the rabid Dr Furhman would not be (correctly) recommending DHA supplementation (along with B12) to avoid brain collapse on veg*n diets. Actually, this link is quite funny when cited by Mc-Starch-Dougall:
"There is no evidence of adverse effects on health or cognitive function with lower DHA intake in vegetarians"
Well, I found it amusing. It's almost the converse of the neurological truism which states that being concerned about having a neurodegenerative disease probably means you don't actually have one.
Anyhoo. Away from the coast we have to get our DHA from animals (or buy algae derived supplements). They get it from grass. There is DHA present in adipose tissue of herbivores just as much as it is present in lipid membranes of their cells. My suspicion is that DHA is a signal to your metabolism that you have just eaten animal fat, from an animal who's food chain starts with grass [or algae]. The more fat you eat, the stronger the signal. We do not need much DHA overall for our brains as it is well protected in this site, but we might well be using it at low levels as a [G-protein coupled receptor sensed] signal to target metabolic adaptation to process fat. So is McDougall correct that veg*n "brain" tissue is OK, despite their periphery being depleted? Shrug.
Fish oil supplements? Well, using our "dietary fat is here" marker to pharmacologically modify some perceived CVD risk factor, without the appropriate change in source of metabolic fuel supply, looks to me to be of very limited value. Large intervention trials do show some benefit from omega 3s provided you do your stats well enough, you have a large enough population to pick up a very small effect and you give a high enough dose. But they do not seem to be any sort of panacea. Especially of you are avoiding dietary fat while "faking" the signal that you have eaten dietary fat...
This is not exactly surprising when you try to pick the likely physiology apart. I like the concept of DHA as an animal fat signal.
Peter
Final thought: Do we need omega 3 PUFA at anything above the most minimal levels if we are in saturated fat based ketosis? Of course I don't know. But the signal to cope with starvation is palmitic acid (physiological insulin resistance), not DHA. I live in starvation mode, not on a mixed diet with only intermittent access to healthy ruminant fat. I have long wanted to look at the selective release of FFAs from adipocytes in extended starvation. My suspicion is that in the early days after glycogen depletion palmitic acid is preferentially released over other lipids, PUFA are not needed/wanted. By a few weeks all the palmitate is gone and whatever is left then gets released. People like David Blaine suddenly start to feel weak, wobbly and are probably hypoglycaemic once they run out of palmitate and have to release less saturated fats. Two to four weeks if you carry some spare weight. Sauer's rats had only ever been fed a low fat omega 6 based diet and had no serious palmitate reserves, PUFA release came early for these.
In the lab situation rapid hepatoma tumour growth needs either arachidonic or linoleic acids. The acids must be taken up in to the hepatoma cells, they must be acted on by lipoxygenase to produce 13-hydroxyoctadecadienoic acid, better known as 13-HODE. 13-HODE appears to be the mitogen which promotes rapid cancer growth. 13-HODE looks like a repair signal gone wrong in cancer cells. Omega 3 fatty acids block omega 6 fatty acid uptake in to hepatoma cells. That's all well and good but the reason I got in to this paper was omega 3 PUFA signalling, rather than those omega 6 issues...
OK, Sauer starts to give some pointers on the function of omega 3 fatty acids in health. That's interesting, as I'm no great lover of any sort of PUFA when I view them from the Protons perspective, yet omega 3s seem to come out pretty well, certainly at low doses. You know my fall back, omega 3 PUFA don't always behave like omega 6 PUFA because they get used as signalling molecules blah blah blah. My own inability to tie the molecular structure of omega 3s to their clinical effects is very frustrating! That they probably act are sites "above" the ETC suggest that they act as what I view as 'high level signals".
Well they do.
The signalling appears to be through a G-protein linked receptor with all of the usual cAMP cascade that follows binding of a ligand to such a receptor. What I found particularly interesting was the effect produced on fat pads of normal rats when EPA (other papers from Sauer suggest all omega 3s act on whichever receptor is involved) was added to the perfusate.
OK, here is a neat little graph taken from here:
This is from fed rats. In the fed state the FFA uptake by the inguinal fat pad of a rat is about 6 mcg/min/gram, white open squares.
Adding EPA at 0.84mmol/l (a bit supraphysiological for EPA but let's let that ride) and FFA uptake by the fat pad drops to zero, or close to zero. Or, in fact, you could argue a suggestion of fatty acid relase, shown as a negative uptake value. Black circles. Fatty acids are not taken up, they end up in the venous effluent in the experiment, plus a little extra.
Whooooah, so do FFAs go through the roof when you take fish oil IRL??
Well no. That's because of this graph from here in the same paper:
Here we have the free fatty acid release from the inguinal fat pad of a healthy rat who has been starved for 48 hours. Fatty acid release is trundling along at about 3mcg/min/gram until EPA is added, again at around 0.8mmol/l. The release of FFAs, in the fasted state, is eliminated. Table 1 in the same paper shows you can get this effect of halting lipolysis in starved rats with under 0.3mmol/l EPA.
Both effects are mediated through a G-protein coupled receptor, ie high level signalling compared to electrons and superoxide in the electron transport chain.
Obviously there are a number of serious problems with this paper but, as a proof of concept, I buy it. I doubt DHA or alpha linolenic acid would work as well (or the group would have used them for this proof of point exercise!) and I think the levels of EPA used produce a very artefactual "switch-like" effect which is probably a graded response. I doubt 0.8mmol/l or even 0.3mmol/l of EPA is exactly physiological but...
Let's suggest that there is a progressive removal of the influence of adipocytes from the FFA flux in/out of plasma as the level of omega 3s in arterial blood increases. Omega 3 fatty acids render adipocytes irrelevant to free fatty acid levels in the plasma.
That is one hell of an idea.
Next we need a brief look at hepatoma cells, again the graph is provided by Sauer and it shows that omega 3 fatty acids, in a G-protein coupled receptor manner, completely turn off the uptake of ALL fatty acids in to hepatoma cells.
If, and it's quite a big "if", the same effects apply to hepatocytes as well as hepatoma cells, we then have a very straightforward mechanism for the protective effects of omega 3 fish oils on hepatic lipidosis. From my point of view this is quite real as there are pretty convincing papers showing that cats, in real life, can be largely protected against the potentially fatal hepatic lipidosis of rapid weight loss by modest doses of omega 3 fatty acids.
Soooo while omega 3s stop the release of all FFAs from adipocytes, they simultaneously stop the uptake of all fatty acids in to the two primary storage organs for fatty acids, adipocytes and liver.
Do plasma FFAs go up or down?
They do, of course, go down. A paradox? Next paper.
Health warning: This paper is so steeped in VLDL and ApoB lipophobia that it makes difficult reading. But there is so little published on FFAs and omega 3 supplementation that it's worth the ondansetron to read it. It's looking at how omega 3 supplements might lower fasting triglycerides, which are the devil incarnate for CVD risk. A huge chunk of VLDL comes from FFAs released from adipocytes and their subsequent repackaging by the liver. Apparently, and I quote from the abstract:
"FO [fish oil] counteracts intracellular lipolysis in adipocytes by suppressing adipose tissue inflammation"
A bit like insulin resistance is caused by "inflammation". Well, maybe it's that simple. They have taken the concept of high level signalling to its 2013 pedestal without looking for basic mechanisms. They have placed the G-protein coupled receptor on to macrophages in the fat pads, which subsequently control the adipocyte lipolysis using cytokines. I haven't checked how good this concept is. Sauer never looked that deeply. Looks a bit modern to me.
Personally I would guess that there are similar receptors on both adipocytes and hepatocytes, but the review does not seem to cover the ability of omega 3s to inhibit general fatty acid uptake by these two tissues. Ah well.
What they do argue is that omega 3 fatty acids upregulate lipoprotein lipase, pretty well whole body. Of course liver and adipocytes ignore this fatty acid bonanza, as above. LPL upregulation is what I needed to know from this paper.
So where do "spare" fatty acids go to? They go to muscles. Upregulated lipoprotein lipase (heralded as the saviour from elevated fasting triglycerides) allows increased lipid release from VLDL to lower those fasting triglycerides. But it's worth bearing in mind that cells do not "see" VLDL, the LPL is on the vascular endothelium and the cells behind the vessel wall only ever receive "free" fatty acids. These are not labelled as from albumin, VLDL or chylomicrons.
Slight aside for later: It seems likely that chylomicrons are going spill their lipids via that same LPL, worth remembering.
The story in the review can be sumarised as omega 3 fatty acids block the release of FFAs from adipocytes and increase the activity of lipoprotein lipase pretty well whole body. VLDL drops, FFAs drop. All is happy in the cardiovascular system. If you believe.
Various "bits" of omega 3s, especially the lipid peroxides of DHA, are signals for mitochondrial biogenesis. I had a paper which specified which lipoxide was most effective but must have missed the "save" button. Mea culpa yet again. There are hints here.
That's a very neat story, which has more than a grain of truth to it.
Why is it like this? What does it mean, physiologcally? Speculation time:
Omega 3 fats come from plants. Mostly from chloroplasts. Where do humans get their omega 3s from? Certainly not from plants. If we did then the rabid Dr Furhman would not be (correctly) recommending DHA supplementation (along with B12) to avoid brain collapse on veg*n diets. Actually, this link is quite funny when cited by Mc-Starch-Dougall:
"There is no evidence of adverse effects on health or cognitive function with lower DHA intake in vegetarians"
Well, I found it amusing. It's almost the converse of the neurological truism which states that being concerned about having a neurodegenerative disease probably means you don't actually have one.
Anyhoo. Away from the coast we have to get our DHA from animals (or buy algae derived supplements). They get it from grass. There is DHA present in adipose tissue of herbivores just as much as it is present in lipid membranes of their cells. My suspicion is that DHA is a signal to your metabolism that you have just eaten animal fat, from an animal who's food chain starts with grass [or algae]. The more fat you eat, the stronger the signal. We do not need much DHA overall for our brains as it is well protected in this site, but we might well be using it at low levels as a [G-protein coupled receptor sensed] signal to target metabolic adaptation to process fat. So is McDougall correct that veg*n "brain" tissue is OK, despite their periphery being depleted? Shrug.
Fish oil supplements? Well, using our "dietary fat is here" marker to pharmacologically modify some perceived CVD risk factor, without the appropriate change in source of metabolic fuel supply, looks to me to be of very limited value. Large intervention trials do show some benefit from omega 3s provided you do your stats well enough, you have a large enough population to pick up a very small effect and you give a high enough dose. But they do not seem to be any sort of panacea. Especially of you are avoiding dietary fat while "faking" the signal that you have eaten dietary fat...
This is not exactly surprising when you try to pick the likely physiology apart. I like the concept of DHA as an animal fat signal.
Peter
Final thought: Do we need omega 3 PUFA at anything above the most minimal levels if we are in saturated fat based ketosis? Of course I don't know. But the signal to cope with starvation is palmitic acid (physiological insulin resistance), not DHA. I live in starvation mode, not on a mixed diet with only intermittent access to healthy ruminant fat. I have long wanted to look at the selective release of FFAs from adipocytes in extended starvation. My suspicion is that in the early days after glycogen depletion palmitic acid is preferentially released over other lipids, PUFA are not needed/wanted. By a few weeks all the palmitate is gone and whatever is left then gets released. People like David Blaine suddenly start to feel weak, wobbly and are probably hypoglycaemic once they run out of palmitate and have to release less saturated fats. Two to four weeks if you carry some spare weight. Sauer's rats had only ever been fed a low fat omega 6 based diet and had no serious palmitate reserves, PUFA release came early for these.
Subscribe to:
Posts (Atom)