I'm interested in how an uncoupling agent, which works by limiting ROS generation from the mitochondrial inner membrane, can be described as being insulin sensitising while causing fat loss. This clearly doesn't make sense: anything which increases insulin signalling should increase insulin action. A core action of insulin is the storage of lipid. Fat loss is synonymous with reduced insulin signalling.
This next paper
BAM15‐mediated mitochondrial uncoupling protects against obesity and improves glycemic control
is one I've talked about before. Today I would like to examine Figure 3 in some detail. This work was done using C2C12 myotubes as muscle surrogates and, as far as I can make out from the methods, the DMEM is high glucose, 25mmol/l when fresh, and changed every other day.
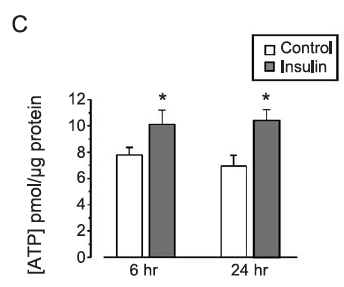
The core marker for insulin signalling in the study was the phosphorylation of AKT, shown in graph B of pAKT 10 minutes after exposure to insulin:
I think we can accept that insulin increases the phosphorylation of AKT, blue columns, but there is only a relatively weak dose response in normal C2C12 myotubes. The pink columns show the effect of 16 hours of pre-incubation with BAM15 before exposure to insulin for 10 minutes. I think we can see a reasonable dose response this time with the highest dose of insulin giving the greatest pAKT increase.
Conclusion: Pre-incubation with BAM15 increases pAKT, a good indicator of insulin signalling. Ergo BAM15 is insulin sensitising.
Well, maybe.
Now it's time to re-label graph B. Digging back in to one of Kevin Hall's papers I found that peak insulin in a normal human being after a high carbohydrate meal was in the region of 1000pmol. So we have 0.5micromol insulin as a 500 times peak physiological value and 1.0micromol is 1000 times peak physiological.
Let's be clear. If we use 1000 times peak human physiological insulin we can further increase the action of insulin to phosphorylate AKT by preincubating with the uncoupler BAM15. Clear cut.
Next we should re-visit insulin-induced insulin resistance from back in 2018 and this paper:
Insulin Resistance Induced by Hyperinsulinemia Coincides with a Persistent Alteration at the Insulin Receptor Tyrosine Kinase Domain
and have a look at Figure 2, graph C, which looks like this
I've added the insulin concentrations, this time in nanomoles, which makes it simple to realise we are looking at 5x, 17x or 170x peak physiological insulin exposure.
Core concept: You cannot increase pAKT above that of modestly supramaximal (5x grey squares) by increasing insulin exposure to lethal overdoses, be that 170x physiological here (black squares) or 1000x physiological as in the BAM15 study. Particularly at the 10 minute mark.
Unless you pre-treat BAM15.
My conclusion is that BAM15 increases the level of pAKT induced by massively supramaximal insulin by reducing insulin-induced insulin resistance. This means that the normal physiological resistance to excessive insulin exposure, ie the refusal to respond further, is blunted. So pAKT goes up.
Again, to clarify: Excessive insulin signalling leads to insulin-induced insulin resistance. BAM15 *reduces* insulin signalling allowing a massive excess of insulin to do a little more phosphorylation of AKT before resistance kicks in. This is a direct consequence of reduced insulin signalling plus supra maximal insulin exposure.
If we were to look at pAKT under conditions of therapeutic uncoupling, it would be decreased. No one in their right mind would do such a study because it would not fit in with the paradigm that insulin sensitisation is a Good Thing. But, if you look hard enough, you can find papers which report this correct finding almost accidentally...
The Mitochondrial Uncoupler DNP Triggers Brain Cell mTOR Signaling Network Reprogramming and CREB Pathway UpregulationThe study used therapeutic levels of DNP (the mice didn't die!) to uncouple the mitochondria of brain cells exposed to physiological concentrations of insulin (ie produced by eating crapinabag mouse chow):
"The protein levels of AKT, p-AKT (Thr308), ERK, and p-ERK were examined by immunoblotting which showed that the activated (phosphorylated) forms of these kinases (p-AKT and p-ERK 42/44) were
reduced in the cerebral cortex at 24 and 72 h after DNP treatment (Fig.
3c–e). Collectively, these results suggest that insulin receptor signaling is suppressed in cerebral cortical cells in response to mild mitochondrial uncoupling."
Aside: I can do exactly the same analysis using metformin. Using a lethal dose of metformin combined with a lethal doses of insulin results in increased peak pAKT.
I
discussed here giving a therapeutic dose of metformin to a real live human with genetically limited insulin signalling. It reduces their ability to insulin signal still further (and pisses them off big time).
End aside.
BAM15 or DNP (or metformin) all work therapeutically to reduce insulin signalling in vivo. This blunts the insulin signalling needed for insulin-induced insulin resistance in vitro which allows a modest increase in pAKT under massive insulin overdose. Therapeutically this reduced insulin signalling allows lipolysis and fat loss.
Ultimately life has to make sense. Mostly it does.
Oh, and semaglutide with its induction of UCP-1 gene expression is no more insulin sensitising than BAM15. Otherwise it wouldn't give fat loss!
Peter