There are researchers within the worlds of both nutrition and cardiology who appear to be very determined to supply a message that failing to eat adequate carbohydrate is a very dangerous choice. They occasionally produce little gems of research.
This paper, which has been sitting on my hard drive for a few years, surfaced on Facebook recently. I gather it has been cited by someone who’s writing requires more ondansetron to read than I currently possess. I’ll just assume it was some sort of “eat LC and the first smidgin of myocardial hypoxia will finish you off” warning, but I’m just guessing and I have every intention of keeping it that way.
The paper itself is very convincing, well written and the protocol extensively justified. The core findings are that a LC diet impairs insulin signalling, depletes myocardial glycogen and results in massive necrosis during reperfusion after a period of myocardial hypoxia. The basic idea is that the lack of glycogen limits substrate for anaerobic glycolysis and failed insulin signalling both impairs glucose delivery from the perfusate and also fails to deliver a number of highly beneficial insulin effects which are independent of GLUT4 translocation.
This is the fate of the LC myocardium. As one of my co workers might say: Dig the hole, choose the coffin.
Obviously, for a LC eater, this is disturbing. The queue for McDougall-ism is over there.
The first thing which I find slightly disturbing is that, in a trial of the Atkins Diet™ (always mention by name), the rats ate more and were significantly heavier than the control rats, within two weeks. I’m not totally certain if I remember correctly, but I thought that the Atkins Diet was used for weight LOSS, not weight gain. Perhaps the authors might have been a little disturbed by this finding too, but apparently it doesn’t need mention. Hmmm.
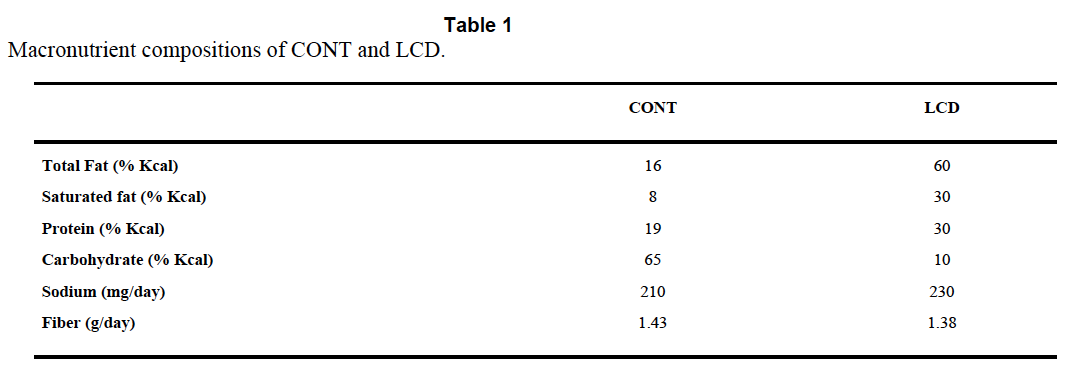
The Atkins™ like diet is TestDiet 5TSY, no longer manufactured. Table I is abstracted by the authors from the full formula provided by Purina and supplies information on a need-to-know basis.
So we know total fat, saturated fat and that “The diets have the same concentrations of essential fatty acids”, i.e. we don’t quite know what the diets were made of. But Purina still will email you a pdf (very promptly) and that gives you this:
There is no mention of Crisco™ by name and no information about the trans fat content, but can you guess how much "Vegetable shortening" is in the control diet? Oh, you guessed!
I know it seems stupid to say this, but if you want to lose weight and/or survive a heart attack, for goodness sake do not choose Crisco or its equivalent as 20% of your calorie intake.
Now, this study does not tell us that a low carbohydrate diet is is good or bad for surviving a heart attack, it's simply not possible to pull that information out due to the lack of control of the variables in the diets. One can only wonder whether the formula of the Atkins Rodent Diet was specifically developed to cause metabolic problems or that somewhere along the line the original Atkins diet suggested a generous consumption of trans fat based vegetable shortening. Maybe I missed this.
I guess you could leave it there and say don't eat vegetable shortening, but there are a whole stack of follow on ideas to this study. There is MASSIVE cardiac necrosis in the LC group of rats. If it is the trans fats, how do they cause this?
One very interesting aspect of the study is the pre-ischaemia depletion of glycogen. These rats are not in ketosis. The doubling of ketone levels from 0.3mmol/l to 0.6mmol/l may well be statistically significant, but is not biologically significant. To go back, yet again, to Veech et al, we need around 5.0mmol/l of mixed ketone bodies to completely replace the insulin signalling system. You can't sidestep insulin resistance with 0.6mmol/l B-OHB. You simply cannot get rats in to a functional level of ketosis with protein at 30% of calories and carbohydrate at 12%. You need carbs near zero and protein limited to < 10% of calories to have rats in nutritional ketosis. Some papers limit protein to < 5% of calories, with minimal carbohydrate.
A period of starvation for rats on the control chow would have tested the hypothesis that it was a lack of glycogen which damaged the myocardium under hypoxia.
My own idea is that the glycogen depletion might be a surrogate for insulin resistance rather than carbohydrate restriction. We have long known that trans fatty acids from partially hydrogenated vegetable oils induce insulin resistance (though not in every study!).
So the next thought is: What sort of insulin resistance?
Are we thinking about an excess of superoxide from complex I, to imitate post prandial hyper caloric insulin resistance? Or are we thinking about the insulin resistance of fasting, when uncoupling results in blunted insulin signalling combined with high oxygen consumption and limited ATP generation?
I like the second idea when applied to this study.
If trans fatty acids, which are structurally similar but not quite identical to saturated fatty acids, allow persistent low level uncoupling outside of the physiological role of the normally structured FFAs it is interesting to speculate that they may continue to allow uncoupling when uncoupling should be utterly and totally banned.
The consequences would be decreased ATP production per unit oxygen consumed. A bit like the findings in Table 3 of this paper. The last column gives you the ATP generated per unit O2 consumed. This would be a disaster under hypoxic conditions. The IF line uses industrial trans fats. The soleus muscle is the mitochondrial dependent one cf the tibialis muscle..
ASIDE: It's worth noting that cytoplasmic ATP is a marked inhibitor of uncoupling but that this is easily overcome by adequate levels of mitochondrial ATP binding within the UCP pore from the end which does not produce a conformational change. Figure 7 gives the details but the main text is really clever stuff (without an agenda, as far as I can see). I'll blog about this paper sometime.
Under this control system the occurrence of falling mitochondrial ATP levels should allow cytoplasmic ATP to immediately shut down the normal uncoupling associated with ketogenic eating and maximise coupled ATP production per unit oxygen when this is needed. The interesting question is whether the uncoupling suspected of transfats persists under low mitochondrial ATP levels. END ASIDE.
You could speculate for hours about what trans fats may or may not do.
This is a very fertile area for idea generation. Ultimately, we don't know what a LC diet based on real Food would do to ischaemic damage in the heart. Maybe it will be as bad as a trans fat based LC (weight gain inducing) diet, maybe less severe.
We are never going to find out what Food does by using "AIN-93G Atkins/Rodent 5TSY" diet based experiments. The rats died in vain, especially as the study buried the possibility of lethal effects from trans fats.
Peter
Wednesday, June 25, 2014
Monday, June 16, 2014
Slim mice which don't fart
Germ free mice are quite interesting. I suppose that the first thing we can say about them is that they don’t have any bacterial fermentation in their gut to produce flatus. As a side issue of some interest is that none of their intestinal mucosal cells ever sees any acetate, butyrate or propionate derived from microbial fermentation of fibre in the gut. They seem rather happy that way. You could also say the same about their liver, it too never sees any SCFA bacterial fermentation products. They stay slim.
Ancient history tells us that germ free mice live rather longer than their more flatulent counterparts. I find this quite interesting.
Of course longevity is a relative term and perhaps ought to be qualified a little. We should actually say that they live longer than conventional mice, provided you feed them. Not feeding germ free mice is quite bad for them, they die under starvation significantly sooner than conventional mice do, at higher bodyweight and with more fat reserves.
That was the state of our knowledge at the end of the last century.
In more recent years the mechanisms for this failure to cope with starvation has become a little clearer. Germ free mice are insulin sensitive. They stay that way pretty much whatever you do to them in terms of diet. They stay that way even if you starve them. That, in terms of survival, is a booboo.
There are at least two techniques available via the gut microbiota which might improve the ability to survive starvation and which are gifted to germ free mice by smearing them with pooh from a conventional mouse. One is endotoxin, a subject I suspect I will come back to. The current one, for this post, is short chain fatty acids.
Bacterial fermentation of fibre produces those miracle agents of gut health and general goodness; acetate, butyrate and propionate. These act through a G protein coupled receptor on enterendocrine cells to promote fat storage. This is the Gpr41 receptor. Needless to say the enteroendocrine cells are the same cells which secrete FIAF, as in the FIAF series of posts. The two are possibly related. Given a little effort we could, by looking at conventionalisation of germ free mice, make a good guess about how much of a mouse’s fat belongs to the mouse and how much belongs to its gut microbiota.
So the fermentation products of bacteria promote fat storage when germ free mice are conventionalised. But no one seems to think that a lack of fat was the reason for germ free mice dying sooner under starvation. So what is the other effect of SCFAs, other than a bigger butt?
The gift of ketosis. Germ free mice are crap at ketosis. It’s not that they can’t do it, it seems to be more like a lack of practice. Acetate and butyrate are particularly ketogenic and hit all sorts of signalling systems in the liver to up regulate ketone generation in conventional mice. Of course germ free mice on standard crapinabag never send acetate to their liver, so their liver never up-regulates the correct PPARs to do ketosis. Putting germ free mice on to a deeply, deeply ketogenic diet teaches them how to make ketones and they become rather good at it.
Under starvation the myocardium of a germ free mouse continues to metabolise glucose, despite free fatty acids being available. Crawford et al consider the myocardium to be fairly representative of many of the glucose using organs in the body. They view ketones as an alternative energy source to glucose under starvation. While no one would argue with this, the possibility which fascinates me is that ketones are turning off glycolysis to spare glucose for the brain when fatty acids alone don’t do this. They appear to be a core component of physiological insulin resistance.
Whether these ketogenic germ free mice are able to extend their time of death to that of starved conventional mice is not a question which any modern ethics committee will allow you to answer today, unless you have a damned good reason. But I suspect the answer is yes.
Peter
Addendum. If you accept that perhaps SCFAs expand your butt via Gpr41 (we are not, after all, germ free mice freshly smeared with conventional mouse faeces) guess which metabolite is a direct antagonist to SCFAs at Gpr41? Clue, it's that the beta hydroxylated derivative of butyric acid. I love stuff that makes sense.
Ancient history tells us that germ free mice live rather longer than their more flatulent counterparts. I find this quite interesting.
Of course longevity is a relative term and perhaps ought to be qualified a little. We should actually say that they live longer than conventional mice, provided you feed them. Not feeding germ free mice is quite bad for them, they die under starvation significantly sooner than conventional mice do, at higher bodyweight and with more fat reserves.
That was the state of our knowledge at the end of the last century.
In more recent years the mechanisms for this failure to cope with starvation has become a little clearer. Germ free mice are insulin sensitive. They stay that way pretty much whatever you do to them in terms of diet. They stay that way even if you starve them. That, in terms of survival, is a booboo.
There are at least two techniques available via the gut microbiota which might improve the ability to survive starvation and which are gifted to germ free mice by smearing them with pooh from a conventional mouse. One is endotoxin, a subject I suspect I will come back to. The current one, for this post, is short chain fatty acids.
Bacterial fermentation of fibre produces those miracle agents of gut health and general goodness; acetate, butyrate and propionate. These act through a G protein coupled receptor on enterendocrine cells to promote fat storage. This is the Gpr41 receptor. Needless to say the enteroendocrine cells are the same cells which secrete FIAF, as in the FIAF series of posts. The two are possibly related. Given a little effort we could, by looking at conventionalisation of germ free mice, make a good guess about how much of a mouse’s fat belongs to the mouse and how much belongs to its gut microbiota.
So the fermentation products of bacteria promote fat storage when germ free mice are conventionalised. But no one seems to think that a lack of fat was the reason for germ free mice dying sooner under starvation. So what is the other effect of SCFAs, other than a bigger butt?
The gift of ketosis. Germ free mice are crap at ketosis. It’s not that they can’t do it, it seems to be more like a lack of practice. Acetate and butyrate are particularly ketogenic and hit all sorts of signalling systems in the liver to up regulate ketone generation in conventional mice. Of course germ free mice on standard crapinabag never send acetate to their liver, so their liver never up-regulates the correct PPARs to do ketosis. Putting germ free mice on to a deeply, deeply ketogenic diet teaches them how to make ketones and they become rather good at it.
Under starvation the myocardium of a germ free mouse continues to metabolise glucose, despite free fatty acids being available. Crawford et al consider the myocardium to be fairly representative of many of the glucose using organs in the body. They view ketones as an alternative energy source to glucose under starvation. While no one would argue with this, the possibility which fascinates me is that ketones are turning off glycolysis to spare glucose for the brain when fatty acids alone don’t do this. They appear to be a core component of physiological insulin resistance.
Whether these ketogenic germ free mice are able to extend their time of death to that of starved conventional mice is not a question which any modern ethics committee will allow you to answer today, unless you have a damned good reason. But I suspect the answer is yes.
Peter
Addendum. If you accept that perhaps SCFAs expand your butt via Gpr41 (we are not, after all, germ free mice freshly smeared with conventional mouse faeces) guess which metabolite is a direct antagonist to SCFAs at Gpr41? Clue, it's that the beta hydroxylated derivative of butyric acid. I love stuff that makes sense.
Sunday, June 15, 2014
Cholesterol: Do chylomicrons clog your arteries? (2)
I'll keep this brief.
The comments on the last post are awash with people trying to help my resident lipophobe out of his lipophobia. This is admirable but misguided and ultimately doomed.
Many years ago I looked at the Copenhagen Heart Study. This observational study generated two hypotheses. One is that chylomicrons kill you. That seems enough for a lipophobe and, if you have this mindset, for goodness sake add carbs to your diet and avoid post prandial hyperlipidaemia. The choice is yours. Go for it. The lack of stress will help you no end.
My own hypothesis is that elevated post prandial triglycerides, here in a population on a mixed diet, is a surrogate for insulin resistance. That is rather similar to an elevated HbA1c under the same circumstances. These are both superficial markers for a failure of complex I of the electron transport chain to effectively deal with the amount of NADH being provided by processing of the diet.
The solution from a Hyperlipid point of view is to concentrate on supplying FADH2 to the ETC via beta oxidation of maximally saturated fatty acids and to minimise NADH input via chronic normoglycaemia, possibly assisted by the inhibition of glycolysis by ketones.
I have no interest in converting lipophobes to lipophiles. We all have our problems, we just have to live with them.
But some gems are coming out of chylomicrons and endotoxin reading. I especially enjoyed this one (among many), all quotes from the same paper:
"Therefore, on the basis of current information, lipoproteins modulate the host response to endotoxin by inhibiting the activation of macrophages, monocytes, and other LPS-responsive cells; promoting the catabolism of LPS by the hepatic parenchymal cells; and inhibiting the response of hepatocytes to pro inflammatory stimuli"
"Early in the course of this work, we found that chylomicron increases the clearance of LPS by the liver while decreasing overall TNF-α production"
"These most recent studies are focused on cells that express the low-density lipoprotein receptor and are critical to the innate immune response to infection, including adrenocortical cells and vascular endothelial cells. The postulated series of events, whereby a foreign molecule (i.e., LPS) serves to both trigger and attenuate a programmed cellular stress response, is unprecedented"
My emphasis.
Now, a functional LDL-C receptor is utterly necessary for the anti-inflammatory effect of the chylomicron/endotoxin complex. If someone's ears don't prick up on this one they have clearly never heard of homozygous familial hypecholesterolaemia. The focus on elevated lipids (sigh) might just have missed the core of the problem, which is the failure to internalise lipoproteins. Interesting idea? It certainly is to me.
Peter
The comments on the last post are awash with people trying to help my resident lipophobe out of his lipophobia. This is admirable but misguided and ultimately doomed.
Many years ago I looked at the Copenhagen Heart Study. This observational study generated two hypotheses. One is that chylomicrons kill you. That seems enough for a lipophobe and, if you have this mindset, for goodness sake add carbs to your diet and avoid post prandial hyperlipidaemia. The choice is yours. Go for it. The lack of stress will help you no end.
My own hypothesis is that elevated post prandial triglycerides, here in a population on a mixed diet, is a surrogate for insulin resistance. That is rather similar to an elevated HbA1c under the same circumstances. These are both superficial markers for a failure of complex I of the electron transport chain to effectively deal with the amount of NADH being provided by processing of the diet.
The solution from a Hyperlipid point of view is to concentrate on supplying FADH2 to the ETC via beta oxidation of maximally saturated fatty acids and to minimise NADH input via chronic normoglycaemia, possibly assisted by the inhibition of glycolysis by ketones.
I have no interest in converting lipophobes to lipophiles. We all have our problems, we just have to live with them.
But some gems are coming out of chylomicrons and endotoxin reading. I especially enjoyed this one (among many), all quotes from the same paper:
"Therefore, on the basis of current information, lipoproteins modulate the host response to endotoxin by inhibiting the activation of macrophages, monocytes, and other LPS-responsive cells; promoting the catabolism of LPS by the hepatic parenchymal cells; and inhibiting the response of hepatocytes to pro inflammatory stimuli"
"Early in the course of this work, we found that chylomicron increases the clearance of LPS by the liver while decreasing overall TNF-α production"
"These most recent studies are focused on cells that express the low-density lipoprotein receptor and are critical to the innate immune response to infection, including adrenocortical cells and vascular endothelial cells. The postulated series of events, whereby a foreign molecule (i.e., LPS) serves to both trigger and attenuate a programmed cellular stress response, is unprecedented"
My emphasis.
Now, a functional LDL-C receptor is utterly necessary for the anti-inflammatory effect of the chylomicron/endotoxin complex. If someone's ears don't prick up on this one they have clearly never heard of homozygous familial hypecholesterolaemia. The focus on elevated lipids (sigh) might just have missed the core of the problem, which is the failure to internalise lipoproteins. Interesting idea? It certainly is to me.
Peter
Thursday, June 12, 2014
Endotoxin Absorption on a High Fat Diet
Housekeeping: Endotoxin is always of some interest to an anaesthetist and the link between endotoxin uptake and high fat diets has been in my thoughts somewhat in recent months. This probably ties in to resistant starch in some way, so the next few posts will probably be random doodles around this subject. And maybe why the Protons thread is fairly well out of data to extend it and another look at brain metabolism, particularly why it doesn't run on fat (or glucose much of the time), all need posts as the time becomes available. Plus Toxic forwarded me a link on B12/folate guidelines which can be downloaded here. As she says, medics may well not read these sorts of guidelines but informed patients certainly should. OK here is the post as was planned.
Endotoxin is nasty stuff. If we want some sort of idea of how nasty it is we just have to look at this juicy quote from Hansen et al from Denmark:
“Harte et al. have reported levels of endotoxin to be between 3.3 and 14.2 EU/mL, but these concentrations are known to increase levels of tumor necrosis factor α (TNFα) in plasma and induce a massive inflammatory response in humans”.
The context of the quote is that these folks are commenting on a paper by Harte et al which described the above levels of endotoxin as being found in human volunteers, which should be nearly lethal. The victims, poor folks, had been coerced in to drinking the sort of amount of whipping cream which might be a lunchtimee snack for myself. And they survived. As do I after such an insult (so far).
What galls Hansen appears to be complete lack of any sort of an inflammatory response to this lethal chylomicron/endotoxin cocktail coursing through the bloodstream of the lipophiles (assuming they enjoyed being paid to drink the cream).
In every group studied, from normal through obese and IGT to diabetic, TNFa falls after a decent glass of whipping cream, albeit in a statistically non significant manner. Endotoxin levels go up, often dramatically and highly significantly.
It’s all in Table 2:

Even in the deepest cesspits of nutritional research no one really expects a half a pint of cream to produce lethal endotoxaemia within a few hours of drinking it. In their response to the comment, which implies that they can’t measure endotoxin correctly, Harte are polite and point out that there is a world of difference between an iv bolus of neat endotoxin (not recommended) and the absorption of endotoxin from the gut in the presence of chylomicrons (welcome to my world).
There are a number of papers showing that apolipoprotein B containing lipid particles are markedly protective against endotoxin, albeit mostly in mouse models. Quite why an oral lipid bolus should automatically load chylomicrons with endotoxin in healthy individuals is another of those fascinating questions which may need a little thought but I think we can reliably say that it would have been eliminated if there is no benefit.
I wrote a post, several years ago, where Greve’s group demonstrated a marked protective effect of a gastric lipid gavage against the effects of haemorrhagic shock, the effects of which are largely related to loss of gut wall integrity, bacterial translocation and endotoxin uptake.
In the high fat world, endotoxin uptake is not what it seems. Lipoproteins neutralise and carry endotoxin. If you need systemic inflammation to maintain research funding you need to be a little more McDevious.
The above little exchange came to mind because Zachary mentioned (in comments on the last post) a study at his university recruiting victims to demonstrate endotoxin uptake after a high fat meal WITH inflammation. You can, of course do this. Technique: "The diet is foods such as TV dinners, meat and cheese". This may be good for getting the right result to secure subsequent funding but it obliterates the fact that a single high fat meal of unadulterated whipping cream is both anti inflammatory and might even improve insulin sensitivity, in the acute setting at least. Just skip the Egg McMuffins.
Peter
Endotoxin is nasty stuff. If we want some sort of idea of how nasty it is we just have to look at this juicy quote from Hansen et al from Denmark:
“Harte et al. have reported levels of endotoxin to be between 3.3 and 14.2 EU/mL, but these concentrations are known to increase levels of tumor necrosis factor α (TNFα) in plasma and induce a massive inflammatory response in humans”.
The context of the quote is that these folks are commenting on a paper by Harte et al which described the above levels of endotoxin as being found in human volunteers, which should be nearly lethal. The victims, poor folks, had been coerced in to drinking the sort of amount of whipping cream which might be a lunchtimee snack for myself. And they survived. As do I after such an insult (so far).
What galls Hansen appears to be complete lack of any sort of an inflammatory response to this lethal chylomicron/endotoxin cocktail coursing through the bloodstream of the lipophiles (assuming they enjoyed being paid to drink the cream).
In every group studied, from normal through obese and IGT to diabetic, TNFa falls after a decent glass of whipping cream, albeit in a statistically non significant manner. Endotoxin levels go up, often dramatically and highly significantly.
It’s all in Table 2:

Even in the deepest cesspits of nutritional research no one really expects a half a pint of cream to produce lethal endotoxaemia within a few hours of drinking it. In their response to the comment, which implies that they can’t measure endotoxin correctly, Harte are polite and point out that there is a world of difference between an iv bolus of neat endotoxin (not recommended) and the absorption of endotoxin from the gut in the presence of chylomicrons (welcome to my world).
There are a number of papers showing that apolipoprotein B containing lipid particles are markedly protective against endotoxin, albeit mostly in mouse models. Quite why an oral lipid bolus should automatically load chylomicrons with endotoxin in healthy individuals is another of those fascinating questions which may need a little thought but I think we can reliably say that it would have been eliminated if there is no benefit.
I wrote a post, several years ago, where Greve’s group demonstrated a marked protective effect of a gastric lipid gavage against the effects of haemorrhagic shock, the effects of which are largely related to loss of gut wall integrity, bacterial translocation and endotoxin uptake.
In the high fat world, endotoxin uptake is not what it seems. Lipoproteins neutralise and carry endotoxin. If you need systemic inflammation to maintain research funding you need to be a little more McDevious.
The above little exchange came to mind because Zachary mentioned (in comments on the last post) a study at his university recruiting victims to demonstrate endotoxin uptake after a high fat meal WITH inflammation. You can, of course do this. Technique: "The diet is foods such as TV dinners, meat and cheese". This may be good for getting the right result to secure subsequent funding but it obliterates the fact that a single high fat meal of unadulterated whipping cream is both anti inflammatory and might even improve insulin sensitivity, in the acute setting at least. Just skip the Egg McMuffins.
Peter
Subscribe to:
Comments (Atom)