This is just a longevity study but it provides insight following on from my last post.
Let's just recap:
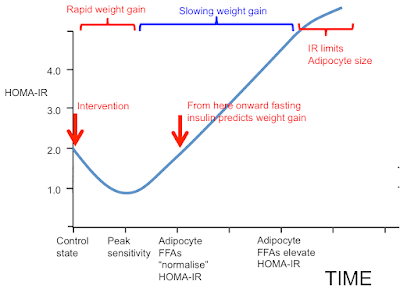
If we start in "control" state and switch to an insulin sensitising diet we have an initial sequestration of fat in to adipocytes and rapid weight gain, red bracket. As adipocytes distend they increase their basal lipolysis, which cannot be suppressed by insulin, and both fasting insulin and post prandial insulin levels must rise. If we fail to observe the initial acute insulin sensitivity phase and limit our observations to the rising insulin resistance phase (blue bracket) we could easily observe that elevated insulin levels are *associated* with future weight gain and assume, incorrectly, that there is causality.
On to the study.
The fat in the control diet is 15% soybean oil, so approx 7% of energy intake as LA. This is not a particularly low LA diet and, as you would expect, fasting insulin levels slowly rise throughout life.
All of the following graphs are extracted from Figure 2 section J, which looks like this
 |
giving this as the control insulin levels:
The high fat (aka insulin sensitising, aka increased LA) diet substituted lard for some of the carbohydrate and was fed ad lib. We don't know how much LA was in the lard, but probably at least 10% of the lard calories were LA. We miss the insulin sensitising hypoinsulinaemic phase as it's long gone by 28 weeks of age:
If we take this graph at face value, at any given age the insulin value is higher in the rats which are fattest, so the logical assumption is that this is causal. QED.
EDIT: I have used my imagination to butcher the above graph back to week eight. Accept or reject at your own discretion!
END EDIT.
But my contention is that these insulin levels are only elevated as a consequence of adipocyte size, ie the hyperinsulinaemia is a secondary phenomenon and could be eliminated by suppressing basal lipolysis, usually using good old acipimox. It works.
But acipimox cannot maintain suppressed lipolysis from distended adipocytes for more than a few hours.
Undoubtedly the most effective way of limiting basal lipolysis is to limit absolute adipocyte size. If we could maintain the adipocytes of the rats fed the insulin sensitising diet at the same size as those of the control fed rats there would be no "masking" effect of elevated basal lipolysis to hide the on going life long insulin sensitising effect of the obesogenic diet.
So they pair fed, by calories, exactly the same "calories" to a third group of rats so that they received exactly the same calories of insulin sensitising food as the chow fed mice eating ad lib.
These rats dis not become fat (I know, I know, heavy calories vs light calories, forgive me) and their weights exactly matched those of the control rats. So now we have rats with normal levels of basal lipolysis for age but no excess through eliminating "over eating" (stop sniggering) the amount of food which hunger, driven by excess insulin sensitivity, demanded in the "green-line" rats.
So the red line is the true insulin sensitivity of rats fed the obesogenic diet when not allowed to superimpose the increased basal lipolysis generated by becoming fat per se:
I hope this post complements the previous post and makes it completely clear that an insulin sensitising diet causes rapid sequestration of fat in to adipocytes. This lost fat will a) make you hungry, b) distend your adipocytes, c) increase their basal lipolysis and d) this latter will cause systemic insulin resistance.
This is a very basic and very simple to understand concept.
Aside: d) is core to limiting caloric ingress to cells when FFAs are available in the presence of insulin/glucose, which should not occur. You know, as in
which is a fundamental paper for understanding hyperinsulinaemia. Ignore at your peril. End aside.
What happens to make adipocytes insulin resistant (a late change in the words of Bill Lagakos, and I agree) is much, much trickier and then we're slipping in to Nick Lane territory.
Peter