My apologies for disappearing off of the blog, after the trip to Alicante and subsequent half term I got out of the habit of blogging and let the fructose thread go cold. I'll get back to it at some stage but this is what is currently making me think. Here we go.
I have a certain specific outlook on life which suggests that insulin sensitivity facilitates fat storage and insulin resistance limits fat storage.
The two anchor points to this world view are here
I think it is fairly reasonable to suggest that this is not a particularly popular point of view and that it may simply reflect my personal biases and my very selective view of the literature. While there are rodent data to support it, the overwhelming evidence from human studies is that insulin resistance/fasting hyperinsulinaemia are strongly associated with future weight gain. I'll cite the below study (let's call it the Odeleye study after the first author) in particular as it too is derived from the Pima Indians and actually mentions the second of my two anchor studies, link courtesy of Gabor Erdosi via Twitter
This is merely the tip of the iceberg and Gabor has many, many studies which show exactly the same finding. I'll stick a list of them down at the end of this post but we only need one for the purpose of my current discussion, the others give a flavour of how universal the finding is. I've not read all of Gabor's links but the titles alone give you the basic gist of their findings. I chose the Odeleye example as it's a Pima study contradicting my own favourite Pima study. It also leads us on to ventromedial hypothalamic lesions in rats, which I like. And the authors are a bit dodgy, which I also like. But there is a vast literature supporting their findings.
So here is the paradox:
Protons says:
Excessive insulin sensitivity facilitates excessive fat storage
and conventional wisdom says:
Insulin resistance makes you hyperinsulinaemic and this hyperinsulinaemia overcomes insulin resistance to facilitate excessive fat storage.
If we set this up as a simple dichotomy and assess evidence by the kilogram (once upon a time studies were published in *paper* journals. Getting a copy involved going to a library and paying 10p a page to photocopy them. You could assess likelihood of Truth by the weight of the paper used to publish. The "weight" of the evidence... Showing my age here) then clearly insulin resistance precedes weight gain. All one needs is insulin hypersecretion to overcome insulin resistance and you have obesity.
How does one tease out the explanation, especially when both directly contradictory concepts have the potential to be correct?
Let's go to the Odeleye Pima study and see what we can find. Here is this line in their discussion:
"However, our results are in contradiction to those obtained in adult Pima Indians in whom insulin resistance and high insulin secretion were associated with a lower weight gain (7,8). Reasons for the discrepant results among studies in children and adults remain to be clarified."
which is as true today as it was in 1997.
In rodent derived support of their findings in Pima children they mention
"These results [in Pima children] are consistent with those in animal studies in which hyperinsulinemia precedes an increase in body weight. In rats, lesions of the ventromedial hypothalamus result in hyperinsulinemia before excess weight accumulation (26)."
giving us, as ref 26, this study:
Unfortunately this study says nothing of the sort. From the abstract
"One week after the lesions, total glucose metabolism was more sensitive and responsive to insulin than in age-matched controls."
"One week after the lesions, total glucose metabolism was more sensitive and responsive to insulin than in age-matched controls."
and from the discussion
"Thus, for a similar increase in plasma insulin levels (i.e., +100μU/ml over basal insulinemia) VMH-lesioned rats actually showed a 92% reduction on hepatic glucose production, while such percentage inhibition was only 46% in control rats (Fig. 1)."
"Thus, for a similar increase in plasma insulin levels (i.e., +100μU/ml over basal insulinemia) VMH-lesioned rats actually showed a 92% reduction on hepatic glucose production, while such percentage inhibition was only 46% in control rats (Fig. 1)."
Aside: Also, by six weeks this enhanced ability to suppress hepatic glucose output of week one was severely compromised in the VHM rats. Secondary to adipocyte distension and increased FFA release in my opinion. It is essential to distinguish primary from secondary effects. End aside.
and from Table 1 we have
This is the best rodent study that Odeleye's group could find. I'm afraid their contention that hyperinsulinaemia precedes weight gain is simply not supported by the study they provide. Fasting insulin of 111μU/ml is neither biologically nor statistically different from 94μU/ml.
Of course they could have cited
Molecular and metabolic changes in white adipose tissue of the rat during development of ventromedial hypothalamic obesity
Molecular and metabolic changes in white adipose tissue of the rat during development of ventromedial hypothalamic obesity
which was available at the time and is even less supportive of their statement
but they didn't. I said they were a bit dodgy! Made me giggle anyway.
There are a number of other fascinating snippets in the rodent studies but I'm wandering and so will try to get back on topic.
Just before I move on to what is actually happening I'd like to throw in the insulin section of Figure 1 from my first anchor study. Pre-obese people are particularly insulin sensitive. I've always cited Table 2 which has HOMA-IR scores to show this. It is quite possible to argue that these fasting data do not represent the dynamic effect on insulin levels in the period after a meal and that post prandial hyperinsulinaemia could easily negate the effect of the fasting values. Luckily for my position the study also fed a mixed meal and tracked insulin levels for six hours following the meal. Like this:
The pre-obese, excessively insulin sensitive people are consistently and significantly hypoinsulinaemic cf controls for at least six hours after a mixed meal. Nice.
Summary:
People at significant risk of obesity are consistently hypoinsulinaemic cf controls, in both fasting and post prandial states.
You can model this by inducing an hypothalamic lesion in rats.
You can model it by high level neonatal MSG exposure in rats
You can model it by the feeding of linoleic acid in rats.
What's happening?
This is my summary:
The time scale might be in weeks for a rodent model changed from chow to a high linoleic acid diet or years for an human neonate given his first bottle of soybean oil based infant formula soon after birth. The intervention might be even further back if his mother has been heeding a cardiologist's advice to limit saturated fats and substitute with polyunsaturates.
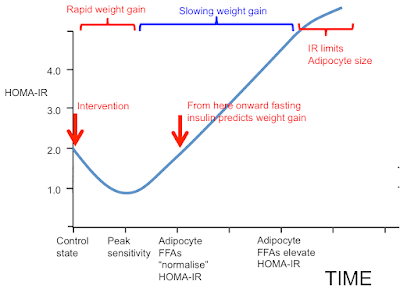
Okay, that's really the end of the post but here are those human observational studies. My assumption is that they are *all* observing the blue bracketed section of the graph labelled "Slowing weight gain".
I have absolutely no doubt that their data are accurately reported. I have serious doubts that any of the authors could explain the left hand end of my graphic. Which is also correctly describing reality. Any hypothesis must explain all the data. Both aspects are correct.
All links via Gabor, much appreciated.
Insulin resistance as a modifier of the relationship between dietary fat intake and weight gain _ International Journal of Obesity
Long-Term Change in both Dietary Insulinemic and Inflammatory Potential Is Associated with Weight Gain in Adult Women and Men
A longitudinal study of serum insulin and insulin resistance as predictors of weight and body fat gain in African American and Caucasian children
Ten-year weight gain is associated with elevated fasting insulin levels and precedes glucose elevation
Pre-teen insulin resistance predicts weight gain, impaired fasting glucose, and type 2 diabetes at age 18–19 y: a 10-y prospective study of black and white girls
Acute Postchallenge Hyperinsulinemia Predicts Weight Gain: A Prospective Study
Long-Term Change in both Dietary Insulinemic and Inflammatory Potential Is Associated with Weight Gain in Adult Women and Men
Insulin resistance as a modifier of the relationship between dietary fat intake and weight gain
Peter




8 comments:
paradoxes, my favorite!
"Any hypothesis must explain all the data." I wish more people remembered that :P
Welcome back, Peter!
What do you make of this? It's similar to the study you've been relying on, comparing nondiabetic individuals with either no history or a family history of diabetes. Fasting levels were more or less the same, but they found that the individuals with a family history of diabetes had less beta oxidation after a high fat meal. They also found that while glucose levels were similar after a high carbohydrate meal, that the individuals with a family history of diabetes required far more insulin.
https://diabetesjournals.org/diabetes/article/56/8/2046/14374/Impaired-Fat-Oxidation-After-a-Single-High-Fat
Hi Peter,
I can't shake the feeling that the increased insulin is simply a method to achieve an increase in H2O2 production so that the cell can breathe. In fat burning, H2O2 production is via glutathione converted to NADP+ for the NADP+ dependent isocitrate dehydrogenase 2, which produces CO2 to allow exchange for O2. Stopping IDH2 leads to obesity in mice, lack of NADP+ will stop IDH2 so also lead to obesity, so will glutathione deficiency and peroxide deficiency. Thus, working insulin will allow the cell more to breathe and get less fat if everything is OK with plenty of O2. If redox chain is not working, it causes insulin resistance but I would call it H2O2 resistance because this signal is not working and the only way out is produce lactate and fat, so tissue hypoxia could be more real then we think. Expression of HIF1 in obese adipose tissue strongly supports this. What do you think?
https://www.nature.com/articles/s12276-020-0379-z
Jaromir
Hi Matt, It simply depends where you are on the curve. The hyperinsulinaemia to produce normoglycaemia will still sequester lipid even on the rising part of the insulin curve. People with systemic insulin resistance still have excessively insulin sensitive adipocytes until very late in the game.
Jaromir, yes, undoubtedly any concept insulin signalling is a superficial overlay on to the core ROS signalling system. But superficiality is sometimes needed to try and convey concepts which seem to be counter intuitive to many people...
Peter
How do we define insulin resistance? Is it hyperinsulinemia? (And if yes, round-the-clock or just postprandial?) Or do we define it by "some insulin mediated biochemical reactions don't work as they do in healthy people given the same insulin levels"? Most papers just seem to base it on HOMA, and I'm not sure that this is a good metric. I believe we have to break it down which pathways are affected in which way.
I am on thin ice here as my knowledge is concerned, so all this may be complete nonsense. Feel free to shoot it down if that's the case :)
Let's say we have someone where the insulin signalling is just fine in most pathways (say, as far as adipocyte fat storage/release are concerned), but glycogen synthase is impaired for some reason (https://doi.org/10.2174/1573399810602040375). Let's say that person sticks mostly to a low carb diet, so everything is fine. But consume any significant amount of carbs, glucose can't be stored as glycogen, so the liver has to work overtime to convert the glucose to fat -> rapid weight gain. HOMA might not be signficantly increased, but we would see very much elevated insulin levels after a carby meal.
So at the very least I'd distinguish between insulin levels in the absence of external glucose (fasting insulin levels) and postprandial insulin levels. I believe HOMA-IR measures the former but not always the latter. And even though both might correlate strongly after a few decades on a western diet, I'm not sure the correlation is universal.
Hi Frunobulax,
Personally I think we are using the one term to describe a number of quite different phenomena. The simplest might be the inability to suppress lipolysis in adipocytes, ie basal lipolysis, ie the proportion of large adipocytes in the body/visceral fat, ie an euglycaemic hyperinsulinaemic clamp. That is not the same as CoQ depletion by a statin drug or glutatiolation of complex I to limit ROS generation.
Also the insulin resistance of fasting will disappear over 30 minutes if you infuse glucose/insulin, so time course matters. Ditto pre/post prandial.
I have other examples but you get the flavour.
Will cross post this to another comment thread as karl made a related point.
Peter
Discuss:
Frunobulax: "How do we define insulin resistance? Is it hyperinsulinemia? (And if yes, round-the-clock or just postprandial?) Or do we define it by "some insulin mediated biochemical reactions don't work as they do in healthy people given the same insulin levels"?
Frunobulax I like your second proposed definition. My understanding of these things is often confused but what seems most relevant to my experiences is expressed in this paper:
"Insulin’s direct effects on the liver dominate the control of hepatic glucose production"
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1359060/
Also my experience is, if "Insulin sensitivity makes you fat. Insulin resistance makes you fat.", nonetheless if the one type of insulin production/sensitivity matches a related version of insulin resistance then you neither gain nor lose weight. There are two things you could call insulin sensitivity implied there, one being the standard definition of the ability of some cell to be affected by insulin, another being the capability of the beta cells to produce insulin in response to stimulation. I don't care so much about gaining or losing weight but I do care about possible hyperglycaemia and the implied hyperinsulaemia, or hypoinsulaemia.
Peter: "... the insulin resistance of fasting will disappear over 30 minutes if you infuse glucose/insulin, so time course matters. Ditto pre/post prandial."
This is one if many information rich sentences which has me slightly befuddled ( easy to do). Could you expand on that a little?
Post a Comment