Here is a schematic illustration of the generation of lactate from glucose as suggested by Schurr, ready for doodling on:
In more artistic style, from Figure 1 in Schurr's paper, it looks like this:
If we look at the more traditional view of glycolysis to pyruvate with a deficit of NAD+ which must be made up by the mtG3Pdh enzyme we have something which looks quite like this:
The concept here is that mtG3Pdh is being used to regenerate the NAD+ which is essential for glycolysis to proceed to pyruvate. Of course, this concept becomes obsolete as soon as we adopt the lactate hypothesis. I tend to view reality as a combination of two pathways like this:
Normal function is via lactate with NAD+ and NADH in perfect balance. The generation of NAD+ from NADH by mtG3Pdh reduces the redox potential of the cytoplasm so the drive to generate lactate, while NADH is in short supply, becomes unhelpful or frankly impossible. For each NADH utilised by mtG3Pdh it is necessary to abort glycolysis at pyruvate, saving the one equivalent NADH by terminating it here rather than at lactate.
It's not that mtG3Pdh replenishes essential NAD+, it's that its consumption of NADH obliges termination of glycolysis at pyruvate. So is mtG3Pdh doing, if the traditional view is a load of bollocks?
Insulin signalling:
Under conditions of high glucose throughput, with insulin signalling, glycolysis will terminate at pyruvate in proportion to the activation of the glycerophosphate shuttle. Notice the two blue arrows signifying two actions of insulin. If we go back to Veech once more, with his isolated heart preparations, we have these two very specific actions of insulin:
One is an increase in glycogen synthesis. Flooding cardiac myocytes with insulin puts every GLUT4 possible on to the cell surface and intracellular glucose approaches that of the perfusing buffer, in this case 10mmol/l. The cells have absolutely no use for this much suddenly available glucose and the simple solution is to divert it to glycogen. The use of intensive insulin therapy in humans with T2D always improves non-oxidative glucose disposal. It goes to glycogen. They get fat too of course. I guess we could quote Veech's abstract:
"The administration of saturating doses of insulin to the glucose perfused, working rat heart acutely increased activity of the glucose transporter 4, GLUT 4, in the plasma membrane (equilibrating extracellular glucose and intracellular [glucose]), activated glycogen synthase (stimulating the rate of glycogen synthesis), and increased mitochondrial acetyl CoA production by the pyruvate
dehydrogenase multienzyme complex. Unexpectedly, insulin increased cardiac hydraulic work but decreased net glycolytic flux and O2 consumption, improving net cardiac efficiency by 28%".
The second arrow points to the mitochondria. Insulin acts to produce a covalent change in the ETC proteins which improves ATP generation per unit oxygen consumed.
In Veech's isolated rat heart preparation insulin REDUCES glycolysis, increases glycogen synthesis and increases mitochondrial ETC efficiency. It's all in the paper on the link above and in assorted related papers, many of which are free full texts and cover the basics. And not so basics.
Veech was looking at combinations of glucose, insulin and ketone bodies. Unlike myself, he is no lover of fatty acids. Ah well. So the buffer used had no fatty acids at all. Now, what do we think insulin will do to fatty acids? It has already maximised the efficiency of the ETC and shunted excess glucose to (harmless) glycogen.
Does insulin facilitate or inhibit the oxidation of fatty acids? The process is just like the diversion of glucose to glycogen:
Once upon a time, before insulin kicked in, CPT1 was used to supply fatty acids to the mitochondria for beta oxidation. Given the increased efficiency of ox phos under insulin, the fate of a significant proportion of fatty acids presenting for beta oxidation is to meet a blockade at CPT1 and to be diverted towards re esterification to triglycerides in the cytoplasm.
I wouldn't suggest that intramuscular triglycerides cause insulin resistance per se. But derivatives there-of do appear to do so and there is certainly an association between intramyocyte triglyceride accumulation and insulin resistance, particularly in non athletes.
This all well and good. Things become more interesting when we take a mtG3Pdh knockout mouse, which cannot signal through mtG3Pdh, and make it run. And run. And run. Until exhaustion, whenever that might be. Of course we also have a rather popular drug to inhibit mtG3Pdh which might mimic the knockout situation. Perhaps for the next post.
Peter
Wednesday, October 28, 2015
Wednesday, October 14, 2015
Protons (37) full glycerophosphate shuttle knockout mice
Just a short post on this paper:
Lethal Hypoglycemic Ketosis and Glyceroluria in Mice Lacking Both the Mitochondrial and the Cytosolic Glycerol Phosphate Dehydrogenases
The glycerol-3-phosphate shuttle is composed of a cytosolic G3Pdh which actually hydrogenates dihydroxy acetone phosphate to glycerol-3-phosphate, using NADH, and a second, mitochondrial version, which does the actual dehydrogenating reconversion back to dihydroxy acetone phosphate. Assuming things are going in the most usual direction.
Lab models of mice with knockout of either part of the glycerol-3-phosphate shuttle are very interesting and are long term survivors, to be discussed another day. Today I’d just like to think about those mice with a complete knockout, deleting both components. They die at a few days of age with an array of problems, lethal hypoglycaemia being the end crisis.
At this stage of life they are being fed a very high fat, low carbohydrate diet (mouse milk, it's mostly palmitic acid. That should tell you something!) and they are very heavily reliant on gluconeogensis to maintain adequate glucose levels using glycerol from the milk's triglycerides as the necessary glucose precursor. As the paper says:
“Glyceride-glycerol is an especially important gluconeogenic precursor in the neonatal mouse, because 80% of calories from mouse milk are derived from fat, 16–17% from protein, and only 2–5% from lactose (20, 22–24). Thus total calories available from dietary glycerol ( 4%) equal calories from lactose.”
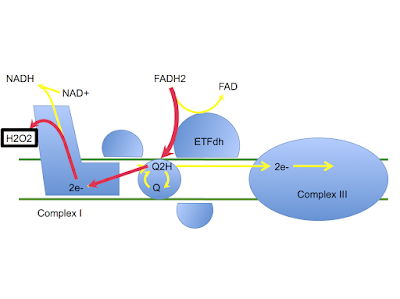
Now, if you are a lab mouse drinking a low carbohydrate diet, should you be insulin sensitive or insulin resistant? If you are insulin sensitive, how much glucose would you like to waste in your muscles, when you only have a limited supply of the stuff in the first place? I would like to suggest that, even in mice, burning glucose for fuel when there is a very limited supply, might not be a survival trait. So insulin resistance, physiological, might be essential for survival. If physiological resistance is essential this is why they have evolved to produce palmitic acid as the main fat in the maternal milk supply. What is needed is a decent input at ETFdh plus and some input at mtG3Pdh to drive enough electrons backwards through complex I to achieve full physiological insulin resistance.
These mice have zero input at mtG3Pdh so are reliant on ETFdh working under palmitic acid to try and achieve this. This clearly doesn't hack it.
The electron transport chain has an ad libitum supply of palmitic acid. The question is:
Without the glycerophosphate shuttle, can the ETC generate reverse electron flow using ETFdh alone to trigger enough superoxide to adequately resist insulin's wasteful usage of precious glucose?
Obviously not. So the second question has to be:
Can pamitic acid at levels in excess of 900micromol/l generate just enough superoxide to allow insulin signalling to commence, without assistance from mtG3Pdh?
I suspect the answer is yes. This generates just enough superoxide (hence H2O2) to allow insulin signalling to commence. Active but inappropriate insulin signalling then triggers a fatal fall in blood glucose as GLUT4s translocate to cell surfaces and glucose drops through them to be squandered irreplaceably. Glucose crashes, the mice die.
It's an extreme model but it makes us think about what might be happening in addition the malonyl-CoA and CPT1 level of signalling and the limited gluconeogenesis discussed so very nicely in the paper.
Peter
Lethal Hypoglycemic Ketosis and Glyceroluria in Mice Lacking Both the Mitochondrial and the Cytosolic Glycerol Phosphate Dehydrogenases
The glycerol-3-phosphate shuttle is composed of a cytosolic G3Pdh which actually hydrogenates dihydroxy acetone phosphate to glycerol-3-phosphate, using NADH, and a second, mitochondrial version, which does the actual dehydrogenating reconversion back to dihydroxy acetone phosphate. Assuming things are going in the most usual direction.
Lab models of mice with knockout of either part of the glycerol-3-phosphate shuttle are very interesting and are long term survivors, to be discussed another day. Today I’d just like to think about those mice with a complete knockout, deleting both components. They die at a few days of age with an array of problems, lethal hypoglycaemia being the end crisis.
At this stage of life they are being fed a very high fat, low carbohydrate diet (mouse milk, it's mostly palmitic acid. That should tell you something!) and they are very heavily reliant on gluconeogensis to maintain adequate glucose levels using glycerol from the milk's triglycerides as the necessary glucose precursor. As the paper says:
“Glyceride-glycerol is an especially important gluconeogenic precursor in the neonatal mouse, because 80% of calories from mouse milk are derived from fat, 16–17% from protein, and only 2–5% from lactose (20, 22–24). Thus total calories available from dietary glycerol ( 4%) equal calories from lactose.”
Now, if you are a lab mouse drinking a low carbohydrate diet, should you be insulin sensitive or insulin resistant? If you are insulin sensitive, how much glucose would you like to waste in your muscles, when you only have a limited supply of the stuff in the first place? I would like to suggest that, even in mice, burning glucose for fuel when there is a very limited supply, might not be a survival trait. So insulin resistance, physiological, might be essential for survival. If physiological resistance is essential this is why they have evolved to produce palmitic acid as the main fat in the maternal milk supply. What is needed is a decent input at ETFdh plus and some input at mtG3Pdh to drive enough electrons backwards through complex I to achieve full physiological insulin resistance.
These mice have zero input at mtG3Pdh so are reliant on ETFdh working under palmitic acid to try and achieve this. This clearly doesn't hack it.
The electron transport chain has an ad libitum supply of palmitic acid. The question is:
Without the glycerophosphate shuttle, can the ETC generate reverse electron flow using ETFdh alone to trigger enough superoxide to adequately resist insulin's wasteful usage of precious glucose?
Obviously not. So the second question has to be:
Can pamitic acid at levels in excess of 900micromol/l generate just enough superoxide to allow insulin signalling to commence, without assistance from mtG3Pdh?
I suspect the answer is yes. This generates just enough superoxide (hence H2O2) to allow insulin signalling to commence. Active but inappropriate insulin signalling then triggers a fatal fall in blood glucose as GLUT4s translocate to cell surfaces and glucose drops through them to be squandered irreplaceably. Glucose crashes, the mice die.
It's an extreme model but it makes us think about what might be happening in addition the malonyl-CoA and CPT1 level of signalling and the limited gluconeogenesis discussed so very nicely in the paper.
Peter
Saturday, October 10, 2015
Protons (38) and ultra low fat once more
OMG, here we go with more doodles. Ah well...
This post is a summary of the ideas which came out of the Protons thread. I’ve been meaning to write it for some time but the trigger was really Denise Minger’s idea of “carbosis”. This has made me revisit ideas I kicked around at the time of the Potato Diet about the systemic level of insulin and revisited within the Protons concept. I guess the idea is to try and work out whether there is any physiologically plausible explanation for the changes seen under ultra low fat eating, under carbosis.
This is my opinion, most of the references are buried in the Protons thread and some ideas I have interpolated from hard facts because they make sense to me. But be aware you are reading an opinion piece.
Cellular insulin response is controlled by superoxide produced at complex I, which exits the mitochondria as H2O2. Small pulses of H2O2 limit the activity of PTP 1B (protein tyrosine phosphatase 1B). Disabling PTP 1B takes the brakes off of the insulin receptor and allows it to autophosphorylate whenever insulin binds and so allows subsequent insulin signalling to take place.
Large amounts of H2O2 inhibit the autophosphorylation of the insulin receptor directly, at several sites, and so cause insulin resistance per se.
So it's pretty obvious that mitochondrial superoxide/H2O2 controls insulin function and subsequent blood glucose levels, and obesity levels if you are a True Believer, which I am. In this post I'd just like to summarise my own personal thoughts on how the electron transport chain, from where much of the the superoxide is produced, behaves under a variety of conditions.
It is quite easy to set up a mitochondrial preparation which can be driven almost completely through complex I. You can feed it on pyruvate/malate. Under these conditions there is essentially zero H2O2 generation, so there is obviously very limited superoxide generation. Think of it like this:
Electrons from NADH simply drop through complex I, fall easily down hill on to the CoQ couple and are handed on to complex III and the rest of the chain. Similarly we can run tissues on pure glucose at modest levels, even if that's not quite how we do it in real life. Many of Veech's papers on ketones used isolated heart preparations which work quite well for quite some time on oxygenated buffer with glucose alone as the sole metabolic substrate, without insulin. So mitochondria can work on pyruvate and isolated hearts can work on pure glucose.
The next piece of input we need to consider is succinate dehydrogenase (SDH), also known as complex II but I'll use SDH as the term in this post. As the TCA turns using one molecule of acetyl-CoA it generates 3 molecules of NADH and one of FADH2, the later being embedded deep within SDH. The FADH2 of succinate dehydrogenase feeds directly to the CoQ couple and reduces it independently of electrons coming from complex I. We have a situation like this:
The TCA is turning and the ETC is accepting input at two points. This is normal physiology and generates very little superoxide, especially while rest of the ETC is well oxidised and so very willing to accept electrons.
With a mitochondrial preparation it is very easy to supply an input of exogenous succinate to SDH without the rest of the TCA cycling in synchrony. This is an experimental situation, only seen in in-tact animals if they are dosed orally with succinc acid esters. This is what happens:
The diagram doesn't make it terribly clear so lets clarify the flow of electrons from SDH, given a sudden massive rise in isolated succinate. Reverse electron flow to superoxide is picked out in red:
Feeding high levels of succinate to a mitochondrial preparation produces massive levels of superoxide. This is not physiology but it can be viewed as a pharmacological demonstration of physiology pushed beyond its normal limits. It's an illustration.
Aside: mitochondria normally oscillate in their function. The TCA drives complex I via NADH (mostly from the early part of the TCA) to the CoQ couple. SDH reduces the CoQ couple independently so opposes this process. Oxaloacetate at the end of the TCA inhibits SDH, facilitating input at complex I, and the whole system goes back and forth as a normal oscillatory process. That's how it is. I’ve no idea why it’s organised this way, but it clearly works rather well! End aside.
But we can say that some degree of reverse electron flow from SDH through complex I will allow small amounts of superoxide generation which will generate modest pulses of H2O2 as far as PTP 1B. This is essential for insulin signalling.
I feel the next step is to look at the situation where glucose is in oversupply, classically after a pure starch meal. Here we need a brake to be applied to the influx of glucose to the cell. In the aftermath of a glucose based meal we would expect insulin to be high, GLUT4s to be active and ox phos to be based on pyruvate (or lactate if you prefer). What is needed is to reduce insulin signalling, limit GLUT4 translocation and so limit glucose ingress to that which is needed by the cell. This is achieved through a side branch of the glycolytic pathway which generates glycerol-3-phosphate. Among the many, many functions of G-3-P, one is to input electrons from NADH to the electron transport chain from the cytoplasmic side of the inner mitochondrial membrane which will reduce the CoQ couple. How much it does this probably depends on the level of metabolites running down through glycolysis. Let's think about a high glycolytic flux, marked reduction of the CoQ couple and see what happens:
The things to note are a large input of cytoplasmic NADH generating marked reverse electron flow through complex I to generate enough superoxide to send H2O2 to inhibit the action of the insulin/receptor complex. It's a simple negative feedback situation and probably produces quite precise control of access of glucose tailored to the needs of the individual cell. There is no pathology here, it's how glucose based metabolism should be controlled.
The next scenario to consider is something like a large ingress of uncontrollable carbohydrate. A find of honey or table sugar. Let's think about it in the complete absence of any fatty acid metabolism. If we have a sudden avalanche of fructose which pours down through glycolysis, what happens? This unstoppable cascade will activate mtG3Pdh much as the excess glucose we have just considered. This should produce a large amount of H2O2 and disable activity of the insulin receptor and produce enough limitation of GLUT4 translocation to exactly limit glucose access by the correct amount to offset the fructose flood. There are other issues from fructose but these are asides. If metabolism is based on pure glycolysis to supply ox phos substrate then fructose can be accommodated by reducing glucose ingress. From the cellular point of view it all balances out and the degree of insulin resistance is at appropriate physiological levels and only occurs while fructose is high, i.e. not for very long. I'll leave uric acid and metabolic syndrome out from the current discussion, needless to say there are issues, for and against.
So I view mtG3Pdh as a balancing act controlling access of carbohydrate to ox phos by controlling insulin signalling through reverse electron transport through complex I. If these are the only components supplying the ETC it seems to be a pretty simple balancing act which might work rather well. On a pure glucose diet some fructose, even quite a lot, is no problem.
Now we have to add in free fatty acids. While beta oxidation generates acetyl CoA and NADH, one molecule of each for each pair of carbon atoms in the chain, they also generate a molecule of FADH2 at the same time. FADH2 is never used as an unbound molecule, here it is stored within electron transporting flavoprotein which delivers FADH2's electrons to the ETC at electron transporting flavoprotein dehydrogenase (ETFdh in the diagram). Much the same as glycerol-3-phosphate at mtG3Pdh, ETFdh reduces the CoQ couple and is adept at driving reverse electron flow through complex I. Low inputs will do nothing or merely generate small pulses of H2O2 at activating levels, high inputs will generate high levels of H2O2 to shut down insulin signalling on the basis that there is plenty of metabolic substrate from fats, minimal glucose is needed, thank you very much. Again, this is pure physiology, I see no pathology in it.
It looks like this:
The step in beta oxidation which produces the FADH2 to drive ETFdh does not occur when the fatty acid being processed presents a double bond at this step. So fully saturated fatty acids generate the maximum amount of FADH2, monounsaturated fats somewhat less and PUFA least of all. The exception is any fatty acids longer than about 18 carbon atoms, these go to peroxisomes rather than mitochondria. So if we go on to consider monounsaturated FFAs we have something like this:
MUFAs produce significantly less FADH2 so less reduction of the CoQ couple and are not used to generate nearly as much insulin resistance as fully saturated fatty acids. This is not surprising as MUFAs are desaturated versions of fully saturated fats and the desaturation process is largely activated by insulin. MUFAs can be thought of as a more carbohydrate tolerant version of saturated fats.
Of course by the time we get to linoleic acid there is even less FADH2 generated and we have very little ability to resist insulin when these are the main fatty acids being oxidised. Omega 6 PUFA facilitate the action of insulin but don't suppress it even when being metabolised in bulk:
So we can view a glucose system in balance with a fatty acid system where the input to the CoQ couple from the fatty acids controls insulin sensitivity to meter glucose access through manipulating insulin signalling or lack there-of.
Saturated fats suit low glucose availability, MUFA suit a mixed diet and PUFA are spawn of the devil. Near zero fatty acids in the mix rely on mtG3Pdh to regulate glycolysis flux.
I suppose we also ought to think of the situation under a large, uncontrolled fructose input through mtG3Pdh occurring at the same time as saturated fatty acids are being oxidised. That gives us this scenario:
Having two inputs reducing the CoQ couple (as well as a little input from SDH) is a perfect recipe for driving extreme reverse electron transport through complex I with the production of completely unreasonable quantities of superoxide and H2O2. This is the scenario of free radical mediated damage combined with serious insulin resistance. D12079B anyone? The problems are less severe with PUFA fats but this leaves us with a different set of problems, not for today. OK.
Before we go on to look at more human based scenarios using low fat diets I guess we need to consider insulin secretion by the pancreas. This is not solely controlled by glucose. In fact I think I’m going to include the only reference in this post as I don’t think I’ve cited this particular paper before, though I may have done so and forgotten!
Suppress most FFAs using nicotinic acid and you will completely abort the secretion of insulin generated by a glucose level of 12.5mmol/l. Replace the FFAs by infusion and you find that insulin secretion is markedly affected by the nature of the FFAs. The longer the acyl chain the more insulin is secreted. The more double bonds, the less insulin is secreted. At a FFA level around 0.1mmol/l, acutely induced, the pancreas will not secrete insulin in response to 12.5mmol/l of glucose. It seems to me that getting FFAs this low is well within the realms of possibility using a near zero fat diet. You are then in to the region of minimal insulin secretion combined with maximal insulin sensitivity. I know it’s a rat model, a perfused pancreas, assorted artificial lipid infusions, but the logic holds well. Insulin secretion and insulin response/resistance are remarkably similar processes.
So hypo insulinaemia with marked insulin sensitivity might well be the hallmarks of carbosis. I won’t reiterate my thoughts about hepatic insulin extraction except I see this as complementary to the reduced secretion under ultra low fat conditions.
As a True Believer I cannot see steady weight loss without reduced systemic insulin levels. Carbosis suggests that this is the real situation.
The phrase is ITIS, we all know what this stands for. Low enough fat can quite simply cripple the pancreas' ability to secrete insulin in response to glucose. Low enough fat and there is very limited ability to generate superoxide levels beyond normal PTP 1B inhibiting levels. If we accept that hyperinsulinaemia is the driving force of metabolic syndrome and all of its sequelae we have, under conditions of extreme fat restriction, the potential for reducing insulin while using a maximal carbohydrate diet. i.e. There is a health benefit to carbosis, possibly major, implausible as it seems.
There. I said it. You cannot argue with the physiology.
I believe this is what Denise Minger might be describing using "carbosis" as the corollary of ketosis. Under both of these conditions there is minimal insulin secretion but under carbosis there is enough insulin sensitivity working through mtG3Pdh to accurately regulate a near pure glucose metabolism. Fructose is no problem as there is plenty of "exchangeable" glucose for use in a substitution manner. Fatty acids have to be very low for "carbosis" to occur at all and it will be degraded far more easily by saturated fats than by PUFA, as per the ETC diagrams above and as per Denise’s examples.
The essential feature is low insulin. This is the commonality with ketosis. If there are going to be any health benefits of carbosis the low insulin will be the driving force.
So, am I a convert? This is not a religion, what I would ask is:
How effective is carbosis in the real world of T2 diabetes?
As Denise comments:
"More than half of those 100 diabetic ricers—63%—actually saw their fasting blood sugar drop by at least 20 mg/dL during the diet. Only 15% had their blood sugar go up significantly. The remaining 22 saw little to no change".
Translation: Blood glucose: 15% of people were f*cked. 22% it didn’t help. 63% could maintain carbosis.
Insulin usage:
"‘Twas a similar story in Insulin Land. Of the study’s participants, 68 entered the scene already dependent on insulin. As the carbs raged on, 21 of those insulin-injecters didn’t have to change their dosage; nine needed an increase (including four people who initially weren’t on any insulin at all); and—again comes the cruel, cruel defiance of prediction—42 slashed their usage significantly. In fact, 18 folks were able to discontinue their insulin entirely. Feasting on white rice. And sugar. And fruit juice".
Translation: Insulin usage: 13% were f*cked. 29% derived no benefit. 58% achieved carbosis.
How does this stack up against rather mild carbohydrate restriction in severe T2 diabetics?
This diagram says it all. It's from Haimoto et al in 2009.
All they did was drop carbohydrate intake to just over 130g/d. No ketosis. Look at the changes for the first 3 months in HbA1c:
No one needed to increase meds. No one failed to drop HbA1c. No one had to start on insulin. Most people dropped their sulpha drugs. The large spike upwards in the second section looks like one of the two drop outs. The other drop out seems to be lost in the variation in maintenance of control over the 3-6 month interval. Bear in mind that 130g/d is a VERY modest approach to low carbohydrate dieting in severe T2 diabetes. No ketosis, just 100% response rate to a modest carbohydrate reduction.
How can you compare carbosis with ketosis, or even mild carbohydrate restriction? It's like comparing boiled rice followed by boiled rice plus table sugar with a char-grilled fatty steak (rib eye is my preferred choice), buttered broccoli on the side plus Optimal ice-cream to follow. With extra double cream if you're losing too much weight.
The biochemistry of carbosis is very interesting. It might help just over a half of people who try it. Its therapeutic use seems to be of dubious relevance when real food can provide results in 100% of people who comply to carbohydrate reduction. It's strictly for the anhedonic out there but even these poor souls should be cautious about finding themselves in the group of 13-15% who end up f*cked, metabolically speaking.
No thanks.
Peter
Tuesday, October 06, 2015
Meet the researchers with the PKCζ deletion
Let's get this straight, right at the start: If you have cancer your outlook is probably worse if that cancer cell population has lost its ability to produce the metabolic regulator PKCζ. It's a bad news deletion.
We are looking at this paper (via David Ramsay, I think):
Control of Nutrient Stress-Induced Metabolic Reprogramming by PKCζ in Tumorigenesis
If you were remotely considering a ketogenic diet as a therapy for cancer management I think you might rightly be interested in the paper. Unfortunately it's quite technical (TL;DR perhaps Heather Buschman?) so perhaps it might be easier to go to the article in ScienceDaily for the take-home message. So initially let's look at the press release, the summary from which says this:
"Many scientists have tried killing tumors by taking away their favorite food, a sugar called glucose. Unfortunately, this treatment approach not only fails to work, it backfires--glucose-starved tumors get more aggressive. In a new study, researchers discovered that the protein PKCζ is responsible for this paradox. The research suggests that glucose depletion therapies might work, as long as the cancer cells produce PKCζ"
There are some pretty sweeping and unsupported statements here. If you actually read the paper itself you will find that there is a mass of information derived from cell culture under extreme (zero glucose, high glutamate) conditions which, while it does allow picking out the details of why PKCζ deletion might be so bad, tells you little about real life progression of cancer.
The group made two attempts to transfer their information from cell culture to something resembling real life. One approach was that they investigated, observationally, humans with colorectal cancer.
They found that in these people the outlook is worse if their particular cancer is PKCζ negative. Fair enough. However, attempted glucose restriction applied to humans with colorectal cancer is not exactly a widespread practice and was not mentioned in these patients. So it seems to be a reasonable assumption that those folks with PKCζ negative cancers are dying at an accelerated rate under glucose replete conditions, not under the conditions used in cell culture experiments. But we don't know for sure, there is no information about the blood chemistry or nutritional management of the patients.
The epic fail in the paper comes with the obligatory mouse model, tacked on near the end.
All you have to do is to mate cancer prone mice with mice which are PKCζ negative and a proportion of their offspring will be cancer prone and either PKCζ positive or negative. You can then test your hypothesis that glucose restriction promotes aggressive growth in PKCζ negative cancer prone mice, whose cancers will clearly be PKCζ negative too.
This should be easy.
Just feed the PKCζ negative mice something like that lovely F3666 ketogenic diet and compare them to those fed standard crapinabag. Obviously the low glucose levels from the ketogenic diet will promote early death in the PKCζ negative group as metabolism switches from glucose to glutamine and the cells develop an aggressive phenotype. The cell cultures say this will happen.
But they didn't do this. They actually fed D12079B vs crapinabag.
What is D12079B, you may ask, that you would use it to show that glucose restriction is Badness for PKCζ negative cancer victims?
D12079B is a typical Western Diet, sucrose/butter derived and is specifically marketed to produce metabolic syndrome in mice. Hyperglycaemia with hyperinsulinaemia. The exact, absolute, reliable opposite of the zero glucose used in all of the cell culture work.
Under glucose excess the animals with PKCζ knockout SHOULD have done at least as well as those on crapinabag, because all the cell culture work showed excess cancer growth during glucose RESTRICTION.
But this didn't happen. Under glucose excess the PKCζ knockouts died fastest of all the groups examined.
Let's just emphasise: At no point did the researchers attempt to limit glucose supply in anything even remotely resembling a live animal.
Does glucose restriction promote and aggressive cancer phenotype through a switch to glutamine metabolism, outside of cell culture?
No one knows. Certainly not the authors of the paper and don't get me started on the press release scribbler.
Personally I'm cautious about ketogenic diets in cancer. I'd expect them to do some good but only time will tell how much good and in which cancers.
But I can see a cop-out in a research paper a mile off. Did they try a few mice on F3666 and fail to report it because F3666 was protective? Or did they simply not dare test their hypothesis? At some stage there was a meeting of the senior researchers where the (extremely expensive) transition to a mouse model was discussed. They did not just stick a needle in a list of diets to choose D12079B. Years of work suggested looking at glucose restriction. They deliberately did the opposite. I shake my head in disbelief.
Peter
We are looking at this paper (via David Ramsay, I think):
Control of Nutrient Stress-Induced Metabolic Reprogramming by PKCζ in Tumorigenesis
If you were remotely considering a ketogenic diet as a therapy for cancer management I think you might rightly be interested in the paper. Unfortunately it's quite technical (TL;DR perhaps Heather Buschman?) so perhaps it might be easier to go to the article in ScienceDaily for the take-home message. So initially let's look at the press release, the summary from which says this:
"Many scientists have tried killing tumors by taking away their favorite food, a sugar called glucose. Unfortunately, this treatment approach not only fails to work, it backfires--glucose-starved tumors get more aggressive. In a new study, researchers discovered that the protein PKCζ is responsible for this paradox. The research suggests that glucose depletion therapies might work, as long as the cancer cells produce PKCζ"
There are some pretty sweeping and unsupported statements here. If you actually read the paper itself you will find that there is a mass of information derived from cell culture under extreme (zero glucose, high glutamate) conditions which, while it does allow picking out the details of why PKCζ deletion might be so bad, tells you little about real life progression of cancer.
The group made two attempts to transfer their information from cell culture to something resembling real life. One approach was that they investigated, observationally, humans with colorectal cancer.
They found that in these people the outlook is worse if their particular cancer is PKCζ negative. Fair enough. However, attempted glucose restriction applied to humans with colorectal cancer is not exactly a widespread practice and was not mentioned in these patients. So it seems to be a reasonable assumption that those folks with PKCζ negative cancers are dying at an accelerated rate under glucose replete conditions, not under the conditions used in cell culture experiments. But we don't know for sure, there is no information about the blood chemistry or nutritional management of the patients.
The epic fail in the paper comes with the obligatory mouse model, tacked on near the end.
All you have to do is to mate cancer prone mice with mice which are PKCζ negative and a proportion of their offspring will be cancer prone and either PKCζ positive or negative. You can then test your hypothesis that glucose restriction promotes aggressive growth in PKCζ negative cancer prone mice, whose cancers will clearly be PKCζ negative too.
This should be easy.
Just feed the PKCζ negative mice something like that lovely F3666 ketogenic diet and compare them to those fed standard crapinabag. Obviously the low glucose levels from the ketogenic diet will promote early death in the PKCζ negative group as metabolism switches from glucose to glutamine and the cells develop an aggressive phenotype. The cell cultures say this will happen.
But they didn't do this. They actually fed D12079B vs crapinabag.
What is D12079B, you may ask, that you would use it to show that glucose restriction is Badness for PKCζ negative cancer victims?
D12079B is a typical Western Diet, sucrose/butter derived and is specifically marketed to produce metabolic syndrome in mice. Hyperglycaemia with hyperinsulinaemia. The exact, absolute, reliable opposite of the zero glucose used in all of the cell culture work.
Under glucose excess the animals with PKCζ knockout SHOULD have done at least as well as those on crapinabag, because all the cell culture work showed excess cancer growth during glucose RESTRICTION.
But this didn't happen. Under glucose excess the PKCζ knockouts died fastest of all the groups examined.
Let's just emphasise: At no point did the researchers attempt to limit glucose supply in anything even remotely resembling a live animal.
Does glucose restriction promote and aggressive cancer phenotype through a switch to glutamine metabolism, outside of cell culture?
No one knows. Certainly not the authors of the paper and don't get me started on the press release scribbler.
Personally I'm cautious about ketogenic diets in cancer. I'd expect them to do some good but only time will tell how much good and in which cancers.
But I can see a cop-out in a research paper a mile off. Did they try a few mice on F3666 and fail to report it because F3666 was protective? Or did they simply not dare test their hypothesis? At some stage there was a meeting of the senior researchers where the (extremely expensive) transition to a mouse model was discussed. They did not just stick a needle in a list of diets to choose D12079B. Years of work suggested looking at glucose restriction. They deliberately did the opposite. I shake my head in disbelief.
Peter
Saturday, September 05, 2015
Protons (36) Glycolysis to lactate
Before we can even begin to think about metformin and mtG3Pdh we have to re examine lactate and glycolysis. It's difficult to over emphasise how interesting this hypothesis is:
Schurr, A. Lactate: the ultimate cerebral oxidative energy substrate?
I've no idea who Avital Schurr is. But he appears to be an anaesthetist, always a plus point, and he has a view of glycolysis which I really like.
I've blogged before on the astrocyte-neuron lactate shuttle and why I, from my own personal viewpoint, consider lactate to be the ideal mitochondrial fuel when reverse electron transport through complex I is best avoided. It behaves like glucose but without easy access to mtG3Pdh. With the exclusion of fatty acids from neurons the only reduction in the CoQ couple other than complex I then comes from complex II, part of the TCA. There is no input from ETFdh or mtG3Pdh. Pure acetyl-CoA, driving mostly through complex I.
The lactate shuttle is controversial.
Many years ago I recall a sketch on a comedy program, probably on Radio 4, where two politicians of irreconcilable views were invited in to the studio to debate the finer points of some policy by throwing half-bricks at each other.
I never really realised this at the time but the lactate shuttle polarises people. Lactate is viewed by many as an utterly useless, rather toxic end product of anaerobic glycolysis. It is a surrogate for hypoxia, hypoperfusion or mitochondrial failure. It is remarkably unacceptable to almost all physiologists that lactate can be a super fuel or even a fuel of any sort at all. Schurr goes through the arguments and the people and the papers and the logical fallacies and how two groups can look at the same data and draw radically differing conclusions. Think LCer vs vegan vs potato head. You look at the same studies but see different explanations......... And you know, we can't all be correct. Lactophobia is an emotional response. Schurr's words are ‘glucoseniks’ vs ‘lactatians’. You really have to read the paper!
The basic argument is that glycolysis always goes to lactate through pyruvate via cytoplasmic LDH 5. Whatever the oxygen availability. It's energetically favourable.
Lactate is then transfered to the mitochondria via a monocarboxylate transporter and fuels the TCA via pyruvate generated from the intra mitochondrial LDH, putatively LDH 1.
Partly this is redox related. Traditionally, using pyruvate:
Obviously, when you run out of NAD+ glycolysis would grind to a halt. But just look at how neat things become if you take glycolysis through to lactate:
Glycolysis, if it runs to lactate, is self sustaining. There is no deficit of NAD+, just an NAD+:NADH cycle. There is no need for the glycerol-3-phosphate shuttle, not for NAD+ regeneration anyway. Obviously, once lactate enters the mitochondrion it gets converted back to pyruvate with the generation of NADH. But this NADH is where it's needed, in the mitochondrial matrix, well away from the nucleus, ready to be processed by complex I.
How neat is that? Edward Edmonds suggested "elegant" as the descriptor. Yes, some hypotheses are so elegant the really have to be correct.
If it is correct you can then start asking questions about what mtG3Pdh is doing (if it's not regenerating NAD+) and how this might fit in with metformin. We're also back to what controls insulin sensitivity and how a cell regulates energy throughput. And fructose. And hyperglycaemia. Lots to think about.
Peter
Schurr, A. Lactate: the ultimate cerebral oxidative energy substrate?
I've no idea who Avital Schurr is. But he appears to be an anaesthetist, always a plus point, and he has a view of glycolysis which I really like.
I've blogged before on the astrocyte-neuron lactate shuttle and why I, from my own personal viewpoint, consider lactate to be the ideal mitochondrial fuel when reverse electron transport through complex I is best avoided. It behaves like glucose but without easy access to mtG3Pdh. With the exclusion of fatty acids from neurons the only reduction in the CoQ couple other than complex I then comes from complex II, part of the TCA. There is no input from ETFdh or mtG3Pdh. Pure acetyl-CoA, driving mostly through complex I.
The lactate shuttle is controversial.
Many years ago I recall a sketch on a comedy program, probably on Radio 4, where two politicians of irreconcilable views were invited in to the studio to debate the finer points of some policy by throwing half-bricks at each other.
I never really realised this at the time but the lactate shuttle polarises people. Lactate is viewed by many as an utterly useless, rather toxic end product of anaerobic glycolysis. It is a surrogate for hypoxia, hypoperfusion or mitochondrial failure. It is remarkably unacceptable to almost all physiologists that lactate can be a super fuel or even a fuel of any sort at all. Schurr goes through the arguments and the people and the papers and the logical fallacies and how two groups can look at the same data and draw radically differing conclusions. Think LCer vs vegan vs potato head. You look at the same studies but see different explanations......... And you know, we can't all be correct. Lactophobia is an emotional response. Schurr's words are ‘glucoseniks’ vs ‘lactatians’. You really have to read the paper!
The basic argument is that glycolysis always goes to lactate through pyruvate via cytoplasmic LDH 5. Whatever the oxygen availability. It's energetically favourable.
Lactate is then transfered to the mitochondria via a monocarboxylate transporter and fuels the TCA via pyruvate generated from the intra mitochondrial LDH, putatively LDH 1.
Partly this is redox related. Traditionally, using pyruvate:
- Glucose + 2 NAD+ + 2 ADP + 2 Pi → 2 Pyruvate + 2 NADH + 2 H+ + 2 ATP + 2 H2O
Obviously, when you run out of NAD+ glycolysis would grind to a halt. But just look at how neat things become if you take glycolysis through to lactate:
Glycolysis, if it runs to lactate, is self sustaining. There is no deficit of NAD+, just an NAD+:NADH cycle. There is no need for the glycerol-3-phosphate shuttle, not for NAD+ regeneration anyway. Obviously, once lactate enters the mitochondrion it gets converted back to pyruvate with the generation of NADH. But this NADH is where it's needed, in the mitochondrial matrix, well away from the nucleus, ready to be processed by complex I.
How neat is that? Edward Edmonds suggested "elegant" as the descriptor. Yes, some hypotheses are so elegant the really have to be correct.
If it is correct you can then start asking questions about what mtG3Pdh is doing (if it's not regenerating NAD+) and how this might fit in with metformin. We're also back to what controls insulin sensitivity and how a cell regulates energy throughput. And fructose. And hyperglycaemia. Lots to think about.
Peter
Monday, August 31, 2015
Confirmation bias is not just in my head
I've been sent a link to this study:
Body fat loss and compensatory mechanisms in response to different doses of aerobic exercise—a randomized controlled trial in overweight sedentary males
Bottom line: Moderate exercise produces equal weight loss to greater exercise. I'll come back to this later.
I commented in the last post that I consider leaving food on your plate is a very reasonable surrogate for experiencing reduced hunger. This shows up as weight loss. When we look at the converse, long term "accidental" weight gain, my perspective is the same. Eating an extra portion is a surrogate for responding to hunger. Long term hunger = Long term weight gain.
Let's say that again:
Fat people are fat because they are affected by hunger. Not gluttons. Not lazy. Hungry.
If people are going to lose weight on a long term basis they can only do this if they are not hungry. If they are not hungry they will reject food, establish an energy deficit and lose weight.
I may believe in CICO, but that CICO is controlled by hunger.
Back to the study. As the authors, struggling for a moment of lucidity, say:
"However, on the basis of the present findings, we propose that the introduction of a moderate dose of exercise may actually lead to an increase in NEAT without any increase in EI resulting in a “bonus effect,” whereas a higher dose of exercise may lead to an increase in EI and, thereby, a degree of compensation and less than expected loss of FM"
As clear as mud. Let's translate and simplify:
“… a moderate dose of exercise may actually lead to an increase in CALORIES OUT without any increase in HUNGER ... whereas a higher dose of exercise may lead to an increase in HUNGER [ie more eating] and … less than expected loss of fat mass”.
Exercise makes you hungry, certainly if you over-do it.
This is the authors' conclusion. I think they are correct. Other explanations are possible but they if miss the hunger component they are not of a great deal of help to anyone who wishes to understand obesity, even if they happen to be factually correct.
Peter
Body fat loss and compensatory mechanisms in response to different doses of aerobic exercise—a randomized controlled trial in overweight sedentary males
Bottom line: Moderate exercise produces equal weight loss to greater exercise. I'll come back to this later.
I commented in the last post that I consider leaving food on your plate is a very reasonable surrogate for experiencing reduced hunger. This shows up as weight loss. When we look at the converse, long term "accidental" weight gain, my perspective is the same. Eating an extra portion is a surrogate for responding to hunger. Long term hunger = Long term weight gain.
Let's say that again:
Fat people are fat because they are affected by hunger. Not gluttons. Not lazy. Hungry.
If people are going to lose weight on a long term basis they can only do this if they are not hungry. If they are not hungry they will reject food, establish an energy deficit and lose weight.
I may believe in CICO, but that CICO is controlled by hunger.
Back to the study. As the authors, struggling for a moment of lucidity, say:
"However, on the basis of the present findings, we propose that the introduction of a moderate dose of exercise may actually lead to an increase in NEAT without any increase in EI resulting in a “bonus effect,” whereas a higher dose of exercise may lead to an increase in EI and, thereby, a degree of compensation and less than expected loss of FM"
As clear as mud. Let's translate and simplify:
“… a moderate dose of exercise may actually lead to an increase in CALORIES OUT without any increase in HUNGER ... whereas a higher dose of exercise may lead to an increase in HUNGER [ie more eating] and … less than expected loss of fat mass”.
Exercise makes you hungry, certainly if you over-do it.
This is the authors' conclusion. I think they are correct. Other explanations are possible but they if miss the hunger component they are not of a great deal of help to anyone who wishes to understand obesity, even if they happen to be factually correct.
Peter
Sunday, August 30, 2015
Confirmation Bias in my head
Sometimes you get a reminder that you suffer from Confirmation Bias.
I do.
This morning I went back and dug out the study from Aberdeen:
Which carried the phrases:
"…participants were offered a fixed energy intake of 2000 kcal/d"
"In the isocaloric study, despite being served food of the same energy content, intake was slightly lower (66 kcal/d) and weight loss greater (7.2 ± 2.3 vs. 4.7 ± 1.0 kg in 4 wk, P less than 0.05), on the HF-LC diet after correction for unconsumed food"
"In the isocaloric study, despite being served food of the same energy content, intake was slightly lower (66 kcal/d) and weight loss greater (7.2 ± 2.3 vs. 4.7 ± 1.0 kg in 4 wk, P less than 0.05), on the HF-LC diet after correction for unconsumed food"
Feed overweight men 2000kcal/d of a mixed diet and they eat it all. Feed the same men 2000kcal/d of a mildly ketogenic diet (roughly ++ on ketostix) and they refuse to eat all of the food. What they reject gives a weight loss surfeit compared to when they were on the mixed diet.
There's no evidence they went to the gym on the quiet but the study protocol probably asked them not to do this. Assuming they have gyms in Aberdeen.
I don't give a monkey's about appetite score by VAS, lack of metabolic advantage in a study using 140g/d of carbs etc. Food left on plate = Not hungry.
How much simpler can it be?
This was triggered by an article linked to by Rose Nunez Smith via FaceAche which piqued my interest and which I read right through to the end. The article kept saying things which made sense and fitted with my view of reality. There was no author on the end so I went up to the top only to find it was Gary Taubes.
Well, it made me laugh.
It's good there are people writing for the NYT Sunday Review who point out what really matters. Bugger any metabolic ward study.
BTW re Aberdeen, with ketones at ++ it would have been interesting to know what the FFA levels were like but the arithmetic cited in the study suggests we didn't have a lot of UCP activity during this few weeks of ++ ketostix eating.
Peter
Tuesday, August 25, 2015
Starchy stable isotopes? I don't think so!
Have a read at this statement from Hardy et al 2015:
“…stable isotope analyses indicate a mainly carnivorous diet for Neanderthals; a wider range of isotopic values have been observed in contemporary Middle Pleistocene H. sapiens (Richards and Trinkaus 2009), indicating that considerable differences in the levels of starch consumption existed between these two species.”
Now, if you read this I think you might be led to believe that stable isotope analysis indicates that Neanderthals were carnivores and H sapiens ate a different amount of starch to a carnivore. I feel the implication of this sentence is that H sapiens ate "more-than-zero starch" during the Middle Pleistocene.
You would believe wrongly. Did you check the reference? No? Naughty. Richards and Trinkaus (2009) actually say this:
“As the method only measures protein intake, many low-protein foods that may have been important to the diet (i.e., high caloric foods like honey, underground storage organs, and essential mineral and vitamin rich plant foods) are simply invisible to this method.”
The data do not deny starchivory. But the data equally do not in any way support its occurrence. Starch, fruit and honey are invisible on stable isotope analysis. This is a gross mis-citation of Richards and Trinkaus by Hardy et al. Never believe stuff like this without checking the refs. Easy when it is a freebie in PLOS. What do Richards and Trinkaus actually say about diets of carnivorous Neanderthals vs H sapiens? Try this:
“There are now enough isotopic data to see patterns in the data, and they show that the Neanderthals and early modern humans had similar dietary adaptations, obtaining most of their dietary protein from animals, although some of the early modern humans obtained significant amounts of their protein from aquatic, and not just terrestrial, sources.”
You can tell H sapiens ate fish because aquatic food chains are long. The longer the food chain the greater the effect visible in stable nitrogen isotopes. They make fish eating carnivores look like hyper-carnivores. That's how they show up in the paper. Had humans eaten any significant amount of protein rich plants (hazel nuts get cited as a possibility) it would show a lower stable nitrogen ratio. There is no evidence for this.
Did early humans consume starch to grow their brain size? Stop laughing! No one knows, certainly to the point where a starchivorous paper has to mis-cite a completely non-supportive paper as being actually supportive of their rubbish hypothesis.
I love it.
Did you hear the one about Jennie Brand-Miller? Passthecream linked to this gem in the comments of the last post. Some things are just too funny not to share. Have a giggle. J B-M is second author on the starch-is-needed-to-grow-brains paper...
Peter
“…stable isotope analyses indicate a mainly carnivorous diet for Neanderthals; a wider range of isotopic values have been observed in contemporary Middle Pleistocene H. sapiens (Richards and Trinkaus 2009), indicating that considerable differences in the levels of starch consumption existed between these two species.”
Now, if you read this I think you might be led to believe that stable isotope analysis indicates that Neanderthals were carnivores and H sapiens ate a different amount of starch to a carnivore. I feel the implication of this sentence is that H sapiens ate "more-than-zero starch" during the Middle Pleistocene.
You would believe wrongly. Did you check the reference? No? Naughty. Richards and Trinkaus (2009) actually say this:
“As the method only measures protein intake, many low-protein foods that may have been important to the diet (i.e., high caloric foods like honey, underground storage organs, and essential mineral and vitamin rich plant foods) are simply invisible to this method.”
The data do not deny starchivory. But the data equally do not in any way support its occurrence. Starch, fruit and honey are invisible on stable isotope analysis. This is a gross mis-citation of Richards and Trinkaus by Hardy et al. Never believe stuff like this without checking the refs. Easy when it is a freebie in PLOS. What do Richards and Trinkaus actually say about diets of carnivorous Neanderthals vs H sapiens? Try this:
“There are now enough isotopic data to see patterns in the data, and they show that the Neanderthals and early modern humans had similar dietary adaptations, obtaining most of their dietary protein from animals, although some of the early modern humans obtained significant amounts of their protein from aquatic, and not just terrestrial, sources.”
You can tell H sapiens ate fish because aquatic food chains are long. The longer the food chain the greater the effect visible in stable nitrogen isotopes. They make fish eating carnivores look like hyper-carnivores. That's how they show up in the paper. Had humans eaten any significant amount of protein rich plants (hazel nuts get cited as a possibility) it would show a lower stable nitrogen ratio. There is no evidence for this.
Did early humans consume starch to grow their brain size? Stop laughing! No one knows, certainly to the point where a starchivorous paper has to mis-cite a completely non-supportive paper as being actually supportive of their rubbish hypothesis.
I love it.
Did you hear the one about Jennie Brand-Miller? Passthecream linked to this gem in the comments of the last post. Some things are just too funny not to share. Have a giggle. J B-M is second author on the starch-is-needed-to-grow-brains paper...
Peter
Monday, August 17, 2015
Sweden's dietitian advice? No thank you.
Hot off the press from Uppsala and Stockholm:
A high energy intake from dietary fat among middle-aged and older adults is associated with increased risk of malnutrition 10 years later.
"Contrary to what was expected, a high energy intake from total fat, saturated fat and monounsaturated fat among middle-aged and older adults increased the risk of exhibiting malnutrition 10 years later. However, this applied only to individuals with a BMI < 25 kg/m2 at the baseline. In conclusion, these findings suggest that preventive actions to counteract malnutrition in older adults should focus on limiting the intake of total fat in the diet by reducing consumption of food with a high content of saturated and monounsaturated fat."
Repeat after me. Association does not prove causation. How anyone dare suggest an experimental intervention on a large subpopulation of their nation based on an observational association within a subgroup of the target population is beyond me. How dare they?
It must be embarrassing to be a dietitian in Sweden nowadays but this sort of intervention recommendation is not going to decrease the stupidity index of mainstream dietary advice.
Hopefully sensible people will continue to ignore them!
Peter
A high energy intake from dietary fat among middle-aged and older adults is associated with increased risk of malnutrition 10 years later.
"Contrary to what was expected, a high energy intake from total fat, saturated fat and monounsaturated fat among middle-aged and older adults increased the risk of exhibiting malnutrition 10 years later. However, this applied only to individuals with a BMI < 25 kg/m2 at the baseline. In conclusion, these findings suggest that preventive actions to counteract malnutrition in older adults should focus on limiting the intake of total fat in the diet by reducing consumption of food with a high content of saturated and monounsaturated fat."
Repeat after me. Association does not prove causation. How anyone dare suggest an experimental intervention on a large subpopulation of their nation based on an observational association within a subgroup of the target population is beyond me. How dare they?
It must be embarrassing to be a dietitian in Sweden nowadays but this sort of intervention recommendation is not going to decrease the stupidity index of mainstream dietary advice.
Hopefully sensible people will continue to ignore them!
Peter
Methyltransferase in methanogens
The archaea developed rather differently from the Ech driven bacteria. I want to look at them from the energetic point of view so it seems reasonable to start with this image from the first of the Life series posts, back in February:
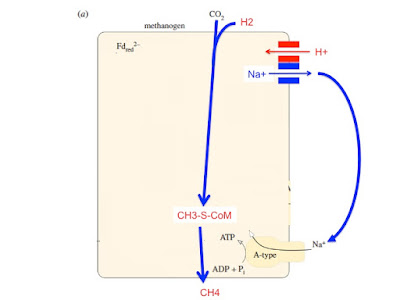
This has CH3-SH (used at two points in the process) driving acetate formation for cell carbon generation. We know this works because Huber and Wächtershäuser demonstrated the abiotic generation of activated acetate from CO and CH3-SH in the presence of an FeS/NiS slurry. No enzymes, no cofactors, no structure. Energetically, it works. I now want to speculate wildly about other uses of CH3-SH in the development of methanogens and the evolution of methyltranseferase, the archaeal alternative to Ech. Let's get rid of the carbon fixation doodles. The location of CH3-SH might need to change as ideas develop:
In the background to the following speculation we still have a FeNi hydrogenase "visiting" a proton channeling pore to generate reduced ferredoxin using a localised low pH region just inside the cell. In the archaea I'm assuming there is no preformed Na+ pump because the protein translocating precursor never jammed up so never pumped Na+ ions. There was no drop in intracellular Na+ and the FeNi hydrogenase never attached firmly to the proton pore in the membrane. Interestingly the methanogens do still have a cytoplasmic FeNi hydrogenase (or NiFe in this illustration) passing electrons down FeS wires. Instead of dropping them straight on to ferredoxin to generate reduced Fd2- using the proton gradient of the cell wall conducted through a membrane pore (as per proto Ech), pairs of electrons are split at an FAD. Half do the up hill job of generating Fd2- and the other half tumble down hill to the easy target of heterodisulphide. Energy from the latter down hill reaction is used to allow the up hill Fd reduction and gets you out of the need for a membrane proton gradient. I do wonder if this is the same FeNi hydrogenase of proto Ech but here diverted to electron bifurcation. This is from Buckel and Thauer's fantastic paper Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation:
That's what happens today. What might have been the core process when metabolism was less refined? Here's a scheme with FeNi hydrogenase (in Hdr, heterodisulphide reductase) using CH3-SH as the electron acceptor for a crude version of electron bifurcation:
Under circumstances of freely available CH3-SH there is no need to conserve sulphur.
The Ni is shown associated with the enzyme which generates all of the biological methane ever produced on earth, methyl coenzyme-M reductase. Nowadays the Ni is bound in the lovely and highly complex coenzyme F430 (an interesting read if you have access):
Here it is in the step producing methane:
Sulphur is no longer a disposable commodity and it is recycled via CoM as a loop in combination with another sulphydryl based coenzyme, CoB.
CoM provides the -CH3 and CoB provides the -H to generate methane. The reaction joins the two coenzymes together to give the mixed, disulphide bridged, heterodisulphide. This is the modern electron acceptor in the Hdr electron bifurcating hydrogenase. It actually accepts a pair of electrons to give CoM-SH and CoB-SH:
The CoM-SH is regenerated to CH3-S-CoM by the methyltransferase shifting a -CH3 from CH3-H4MPT. Ultimately the energetics of the cell is determined by the availability of CH3-SH analogue CH3-S-CoM controlling electron bifurcation at Hdr:
Next let's take out the electron bifurcation system having established a central role for CH3-S-CoM to energetics control and add in the proton port being used to generate Fd2- using the vent H+ gradient:
And add in the antiporter for Na+ ions:
At this point there is some benefit to converting a protein-translocase to a Na+ driven ATP synthase. Consider that it is Na+ ions that are stabilising the membrane section of the translocase so it seems logical to accept a sudden Na+ gradient as the force pushing inwards to reverse the translocase to generate ATP. So we need the A1-A0 ATP synthase adding in to the diagram:
Now a proton gradient is once again driving a Na+ coupled ATP synthase and all is hunky dory for the cell. Excess ATP synthesis can be regulated by CH3-S-CoM inhibiting Na+ antiporting:
When the vent proton gradient fails all that is needed is for CH3-H4MPT to take over from the vent proton gradient. The transfer of the methyl group from H4MPT to CoM is exergonic and is used to drive a conformation change in B12 which ejects the Na+ ion. Lots of detail from Thauer again at The Na‡-translocating methyltransferase complex from methanogenic archaea.
Which simplifies to this:
Giving a speculative journey leading to how we might have ended up here:
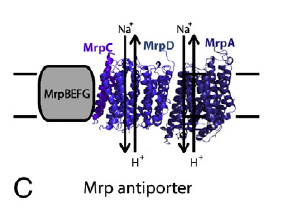
In modern methanogenic archaea Na+ energetics have been carried forward to today. Protons still look like an add on to me. Of course the question posed from here is how similar is the Na+/H+ antiporter of the methanogens to the NouH and NuoL combination in the bacteria, incorporated in to the base of complex I.
Not surprisingly I haven't found anyone crazy enough to float this idea. This is what Thauer has to say about Na+ translocating pores and aspartic acid within the pore channel:
"The second reason for the proposal is that only MtrE has a transmembrane helix with an aspartate residue (Fig. 1), the sequence of this helix in the MtrE subunit from all methanogens being highly conserved: 168-IWGITIGAIGSSTGDVHYGAER-191. An aspartate residue in a transmembrane helix has been shown to be essential for sodium ion translocation as catalyzed by the L-subunit of oxaloacetate decarboxylase from Klebsiella pneumoniae [62]. An aspartate residue is also conserved in the transmembrane helix of the sodium ion-translocating glutaconyl-CoA decarboxylase from Acidaminococcus fermentans and of the sodium ion-translocating methylmalonyl-CoA decarboxylase from Veillonella parva and Propionigenium modestum [63]".
Here is NuoH from complex I with the aspartic acid at D213 picked out in red:
Which rather implies that NuoH, rather than NuoL, was the Na+ part of the antiporter, assuming the membrane portions of methyltransferase and Ech derivatives are distant relatives of the same ancestral protein...
Peter
This has CH3-SH (used at two points in the process) driving acetate formation for cell carbon generation. We know this works because Huber and Wächtershäuser demonstrated the abiotic generation of activated acetate from CO and CH3-SH in the presence of an FeS/NiS slurry. No enzymes, no cofactors, no structure. Energetically, it works. I now want to speculate wildly about other uses of CH3-SH in the development of methanogens and the evolution of methyltranseferase, the archaeal alternative to Ech. Let's get rid of the carbon fixation doodles. The location of CH3-SH might need to change as ideas develop:
In the background to the following speculation we still have a FeNi hydrogenase "visiting" a proton channeling pore to generate reduced ferredoxin using a localised low pH region just inside the cell. In the archaea I'm assuming there is no preformed Na+ pump because the protein translocating precursor never jammed up so never pumped Na+ ions. There was no drop in intracellular Na+ and the FeNi hydrogenase never attached firmly to the proton pore in the membrane. Interestingly the methanogens do still have a cytoplasmic FeNi hydrogenase (or NiFe in this illustration) passing electrons down FeS wires. Instead of dropping them straight on to ferredoxin to generate reduced Fd2- using the proton gradient of the cell wall conducted through a membrane pore (as per proto Ech), pairs of electrons are split at an FAD. Half do the up hill job of generating Fd2- and the other half tumble down hill to the easy target of heterodisulphide. Energy from the latter down hill reaction is used to allow the up hill Fd reduction and gets you out of the need for a membrane proton gradient. I do wonder if this is the same FeNi hydrogenase of proto Ech but here diverted to electron bifurcation. This is from Buckel and Thauer's fantastic paper Energy conservation via electron bifurcating ferredoxin reduction and proton/Na+ translocating ferredoxin oxidation:
That's what happens today. What might have been the core process when metabolism was less refined? Here's a scheme with FeNi hydrogenase (in Hdr, heterodisulphide reductase) using CH3-SH as the electron acceptor for a crude version of electron bifurcation:
Under circumstances of freely available CH3-SH there is no need to conserve sulphur.
The Ni is shown associated with the enzyme which generates all of the biological methane ever produced on earth, methyl coenzyme-M reductase. Nowadays the Ni is bound in the lovely and highly complex coenzyme F430 (an interesting read if you have access):
Here it is in the step producing methane:
Sulphur is no longer a disposable commodity and it is recycled via CoM as a loop in combination with another sulphydryl based coenzyme, CoB.
CoM provides the -CH3 and CoB provides the -H to generate methane. The reaction joins the two coenzymes together to give the mixed, disulphide bridged, heterodisulphide. This is the modern electron acceptor in the Hdr electron bifurcating hydrogenase. It actually accepts a pair of electrons to give CoM-SH and CoB-SH:
The CoM-SH is regenerated to CH3-S-CoM by the methyltransferase shifting a -CH3 from CH3-H4MPT. Ultimately the energetics of the cell is determined by the availability of CH3-SH analogue CH3-S-CoM controlling electron bifurcation at Hdr:
Next let's take out the electron bifurcation system having established a central role for CH3-S-CoM to energetics control and add in the proton port being used to generate Fd2- using the vent H+ gradient:
And add in the antiporter for Na+ ions:
At this point there is some benefit to converting a protein-translocase to a Na+ driven ATP synthase. Consider that it is Na+ ions that are stabilising the membrane section of the translocase so it seems logical to accept a sudden Na+ gradient as the force pushing inwards to reverse the translocase to generate ATP. So we need the A1-A0 ATP synthase adding in to the diagram:
Now a proton gradient is once again driving a Na+ coupled ATP synthase and all is hunky dory for the cell. Excess ATP synthesis can be regulated by CH3-S-CoM inhibiting Na+ antiporting:
When the vent proton gradient fails all that is needed is for CH3-H4MPT to take over from the vent proton gradient. The transfer of the methyl group from H4MPT to CoM is exergonic and is used to drive a conformation change in B12 which ejects the Na+ ion. Lots of detail from Thauer again at The Na‡-translocating methyltransferase complex from methanogenic archaea.
Which simplifies to this:
Giving a speculative journey leading to how we might have ended up here:
In modern methanogenic archaea Na+ energetics have been carried forward to today. Protons still look like an add on to me. Of course the question posed from here is how similar is the Na+/H+ antiporter of the methanogens to the NouH and NuoL combination in the bacteria, incorporated in to the base of complex I.
Not surprisingly I haven't found anyone crazy enough to float this idea. This is what Thauer has to say about Na+ translocating pores and aspartic acid within the pore channel:
"The second reason for the proposal is that only MtrE has a transmembrane helix with an aspartate residue (Fig. 1), the sequence of this helix in the MtrE subunit from all methanogens being highly conserved: 168-IWGITIGAIGSSTGDVHYGAER-191. An aspartate residue in a transmembrane helix has been shown to be essential for sodium ion translocation as catalyzed by the L-subunit of oxaloacetate decarboxylase from Klebsiella pneumoniae [62]. An aspartate residue is also conserved in the transmembrane helix of the sodium ion-translocating glutaconyl-CoA decarboxylase from Acidaminococcus fermentans and of the sodium ion-translocating methylmalonyl-CoA decarboxylase from Veillonella parva and Propionigenium modestum [63]".
Here is NuoH from complex I with the aspartic acid at D213 picked out in red:
Which rather implies that NuoH, rather than NuoL, was the Na+ part of the antiporter, assuming the membrane portions of methyltransferase and Ech derivatives are distant relatives of the same ancestral protein...
Peter
Cholesterol reflectivity at 400nm
I'm interested in paleobiology at the moment, readers might have noticed. Mostly how we might have gotten to where we are now without a Sky Pixie. The ancient past is fascinating. Not only single carbon chemistry under far from equilibrium conditions but big stuff like dinosaurs and cardiologists.
People love dinosaurs. If it's not LDLc levels, it's particle counts or apoB numbers. Am I going to die (yes!)? Will it be from CDV or cancer? Let us examine the entrails of a lipid hypothesis which lies eviscerated here before us. Messy.
How many apoBs can I see???? Do they have purple spots (i.e. does the apoB particle under the microscope have focal regions which are hyper-reflective to photons of 400nm wavelength when irradiated by full spectrum bullshit?).
I think we have to think have to respect the views of the president of the ACC. ACC is the American College of Cardiologists. These folks are wildly intelligent, free thinking, adventurous, swashbuckling promoters of new ideas. The newest idea on view from the president of the ACC is this one:
‘Some prominent cardiologists have questioned the 2013 guidelines, but the ACC and AHA have shown little appetite to return to LDL targets. “LDL may or may not correlate to cardiovascular outcomes,” Dr. Kim Allan Williams, president of the ACC, told Reuters last week1.’
Malcolm Kendrick has an excellent post up on this quote. But let's just say it again, it really is so good:
“LDL may or may not correlate to cardiovascular outcomes”
By whom?
Dr. Kim Allan Williams.
Who is?
President of the ACC.
Which is?
The American College of Cardiologists.
Of course, the statement does leave open the possibility that cardiovascular outcomes MAY correlate to LDL levels. More likely not.
Peter
People love dinosaurs. If it's not LDLc levels, it's particle counts or apoB numbers. Am I going to die (yes!)? Will it be from CDV or cancer? Let us examine the entrails of a lipid hypothesis which lies eviscerated here before us. Messy.
How many apoBs can I see???? Do they have purple spots (i.e. does the apoB particle under the microscope have focal regions which are hyper-reflective to photons of 400nm wavelength when irradiated by full spectrum bullshit?).
I think we have to think have to respect the views of the president of the ACC. ACC is the American College of Cardiologists. These folks are wildly intelligent, free thinking, adventurous, swashbuckling promoters of new ideas. The newest idea on view from the president of the ACC is this one:
‘Some prominent cardiologists have questioned the 2013 guidelines, but the ACC and AHA have shown little appetite to return to LDL targets. “LDL may or may not correlate to cardiovascular outcomes,” Dr. Kim Allan Williams, president of the ACC, told Reuters last week1.’
Malcolm Kendrick has an excellent post up on this quote. But let's just say it again, it really is so good:
“LDL may or may not correlate to cardiovascular outcomes”
By whom?
Dr. Kim Allan Williams.
Who is?
President of the ACC.
Which is?
The American College of Cardiologists.
Of course, the statement does leave open the possibility that cardiovascular outcomes MAY correlate to LDL levels. More likely not.
Peter
Sunday, August 16, 2015
When low fat wins
I think I’ve said before, I’m a calories-in, calories-out sort of person. Nothing as simple as losing a kilo of stored fat for every 9000kcal deficit in dietary consumption (or increase in exercise) of course. This is, as we all know, incorrect and of absolutely no use whatsoever in planning an attempt to generate a normal bodyweight.
I am also very aware that, outside a metabolic ward, it is very difficult to even approximately assess a given person’s level of energy output, assuming they are fully compliant with a fixed composition, fixed caloric input. Which they probably aren’t, much of the time. But calories out will be reflected in many more outputs than can achieved by the limited exercise opportunities afforded during the restrictions of an in-patient metabolic ward study.
As Hall commented, the repeated superiority of weight loss by carbohydrate restriction has always been achieved in outpatient studies, never in tightly controlled metabolic ward studies. He didn’t mention that the advantages from carbohydrate restriction were always achieved under calorically unrestricted circumstances in comparison to calorically restricted alternative diets. I’ll just mention that now.
We can also say, with some degree of certainly, that under very tightly controlled in-patient conditions, extreme dietary fat restriction (less than 8% of calories) produced more stored fat loss than a modest reduction in carbohydrate restriction, provided both groups are rigidly forced to cut calories and to limit their exercise to a specified level, for six days. My own feeling is that this is probably true under the circumstances of the study. It provides a very small piece of data of very limited application to the real world. As Hall writes:
"Translation of our results to real-world weight-loss diets for treatment of obesity is limited since the experimental design [and model simulations] relied on strict control of food intake, which is unrealistic in free-living individuals".
I am very lucky.
I don’t live in a metabolic ward. If the weather is cool outside and I feel warm enough to not need my jacket when I let the chickens out in the morning, so be it. If both the air and water temp are 4degC but there is a four foot swell with clean waves shaping up in First Bay I’m going to be thinking about the roof rack, my playboating kayak and my drysuit. I’m guessing that there are few near freezing surf opportunities in a metabolic ward.
People might also be aware that I rather like fatty acids, especially free fatty acids. These uncouple respiration. Uncoupled respiration, at the mitochondrial level, generates heat and so increases metabolic rate. For people with a heathy interest in cold water kayaking, this has its advantages. Sitting in a metabolic ward eating 140g of carbohydrate per day is not going to increase my free fatty acids to a level were uncoupling is going to feature in my metabolism.
The average person with a BMI of 35 is probably going to be running their metabolism under the Crabtree effect. Increased dependence on glycolysis at the expense of reduced utilisation of mitochondrial beta oxidation. While it is quite possible to immediately and markedly increase fatty acid oxidation, there are limits set by how many mitochondria a given cell possesses. The moderate carbohydrate group did increase their fatty acid oxidation, but not enough to compensate for the loss of carbohydrate available from the diet. This is perhaps most clearly seen in Table 3 where a significant drop in sleeping metabolic rate occurred in the moderate carb group and an actual increase was seen in the very fat restricted group. It's probably why the moderate carbohydrate group had a suggestion of increased protein degradation compared to the very low fat group.
Even thought it was nearly 15 years ago, I can still recall Atkins Flu™ as I switched to deeply ketogenic eating from a fairly reasonable modern diet. The "flu" lasted about 6 days (apologies for exactifying my recall to fit with the study duration!) with further acclimatisation over the next few months. There should be no such problem with increasing glycolysis in the very low fat group if you are already running your metabolism on starch combined with HFCS. It is no major problem to up regulate carbohydrate metabolism when it is your normal metabolic fuel source.
So for the first six days of an enforced, calorically restricted, non-ketogenic diet, cutting fat rules provided the restriction is very, very extreme. Do this long term and you will, of course, fail.
Peter
I am also very aware that, outside a metabolic ward, it is very difficult to even approximately assess a given person’s level of energy output, assuming they are fully compliant with a fixed composition, fixed caloric input. Which they probably aren’t, much of the time. But calories out will be reflected in many more outputs than can achieved by the limited exercise opportunities afforded during the restrictions of an in-patient metabolic ward study.
As Hall commented, the repeated superiority of weight loss by carbohydrate restriction has always been achieved in outpatient studies, never in tightly controlled metabolic ward studies. He didn’t mention that the advantages from carbohydrate restriction were always achieved under calorically unrestricted circumstances in comparison to calorically restricted alternative diets. I’ll just mention that now.
We can also say, with some degree of certainly, that under very tightly controlled in-patient conditions, extreme dietary fat restriction (less than 8% of calories) produced more stored fat loss than a modest reduction in carbohydrate restriction, provided both groups are rigidly forced to cut calories and to limit their exercise to a specified level, for six days. My own feeling is that this is probably true under the circumstances of the study. It provides a very small piece of data of very limited application to the real world. As Hall writes:
"Translation of our results to real-world weight-loss diets for treatment of obesity is limited since the experimental design [and model simulations] relied on strict control of food intake, which is unrealistic in free-living individuals".
I am very lucky.
I don’t live in a metabolic ward. If the weather is cool outside and I feel warm enough to not need my jacket when I let the chickens out in the morning, so be it. If both the air and water temp are 4degC but there is a four foot swell with clean waves shaping up in First Bay I’m going to be thinking about the roof rack, my playboating kayak and my drysuit. I’m guessing that there are few near freezing surf opportunities in a metabolic ward.
People might also be aware that I rather like fatty acids, especially free fatty acids. These uncouple respiration. Uncoupled respiration, at the mitochondrial level, generates heat and so increases metabolic rate. For people with a heathy interest in cold water kayaking, this has its advantages. Sitting in a metabolic ward eating 140g of carbohydrate per day is not going to increase my free fatty acids to a level were uncoupling is going to feature in my metabolism.
The average person with a BMI of 35 is probably going to be running their metabolism under the Crabtree effect. Increased dependence on glycolysis at the expense of reduced utilisation of mitochondrial beta oxidation. While it is quite possible to immediately and markedly increase fatty acid oxidation, there are limits set by how many mitochondria a given cell possesses. The moderate carbohydrate group did increase their fatty acid oxidation, but not enough to compensate for the loss of carbohydrate available from the diet. This is perhaps most clearly seen in Table 3 where a significant drop in sleeping metabolic rate occurred in the moderate carb group and an actual increase was seen in the very fat restricted group. It's probably why the moderate carbohydrate group had a suggestion of increased protein degradation compared to the very low fat group.
Even thought it was nearly 15 years ago, I can still recall Atkins Flu™ as I switched to deeply ketogenic eating from a fairly reasonable modern diet. The "flu" lasted about 6 days (apologies for exactifying my recall to fit with the study duration!) with further acclimatisation over the next few months. There should be no such problem with increasing glycolysis in the very low fat group if you are already running your metabolism on starch combined with HFCS. It is no major problem to up regulate carbohydrate metabolism when it is your normal metabolic fuel source.
So for the first six days of an enforced, calorically restricted, non-ketogenic diet, cutting fat rules provided the restriction is very, very extreme. Do this long term and you will, of course, fail.
Peter
Wednesday, July 29, 2015
Ech
When I draw my doodles of early energetics my standard protocol is to have low pH, high H+, oceanic region at the top. The ocean is above.... I have the vent derived, geothermal, low H+ region at the bottom, that's were the rocks are.
Once people start looking at modern membrane complexes there is no need for such conventions and figures are usually produced the other way up. So when we want to look at modern Ech and derivatives I think I have to turn my proto-Ech doodle upside down like this:
I have, very naughtily, butchered a cartoon of a modern Ech (out of the lovely paper from Efremov and Sazanov) to produce this image of my concept of proto-Ech. As in the doodle, this must use a geothermal proton (not Na+) gradient and does not look to be reversible:
Note that I've altered the black arrows to match my red arrows, showing the generation of reduced ferredoxin from oxidised ferredoxin and the consumption of (primordial) H2 under the influence of oceanic proton flow through the brown membrane protein labelled EchB but which I will refer to from here onwards as NuoH, or a homologue there-of. Their original image of a modern Ech on which I based my proto-Ech is this one:
Again, for various reasons, I will refer to the blue subunit EchA as NuoL. This modern Ech is energy converting, it pumps protons through the blue anti porter homologous to NuoL. All of the arrows are now in their modern pumping direction. The pump is powered by reduced ferredoxin (from elsewhere in the cell) and produces hydrogen as waste. Typical bacteria using this Ech might be E coli, to produce flammable flatus. The source of reduced ferredoxin is any catabolic process. Fermenting starch in the anaerobic colon has predictable results in this department.
An understanding of the initial role for this antiporter is crucial to any logical approach to bioenergetic evolution. Equally interesting are the adaptions of subunit EchB (homologous to NuoH) to changing the activity of NuoL from antiporting to Na+ pumping.
We can now look at a NuoL type antiporter embedded in a membrane without any power supply. It will consume membrane proton potential by allowing protons to move inwards and expel Na+ ions outwards in exchange, like this:
It can do this in a modern bacterium because there is a proton gradient, created by complex I (or any other energy converting complex/hydrogenase), i.e. protons are available to travel down a concentration gradient in to the cell, much as they might have done in primordial times. Na+ ions are forced out against a concentration gradient, in this case because this particular microbe lives in an extremely high Na+ environment. If you delete the Mrp genes for subunits MrpD and MrpA you cripple its antiporting ability. Engineering back in homologous proton translocators from a modern complex I restores the full antiporting ability.This tells us that the modern proton translocators of complex I are still antiporters but their antiporting is kept suppressed. I would suggest by NuoH. Note that the Mrp antiporting complex has no NuoH subunit.
NuoL does appear to be distantly related to NuoH, possibly by very ancient gene duplication. The conservation of form can be seen in these lovely images from Marrreiro et al. The gold ribbon is NuoH and the grey one is NuoL. The channel form is clearly visible. I consider NuoH to be homologous to the original proton ion channel from my proto-Ech doodle and its relative NuoL to be homologous to a prototypical Na+/H+ antiporting derivative.
I think that the initial gene duplication/modification would probably have produced an uncontrolled antiporter which would have produced a dramatic fall in intracellular Na+ concentration. This activated ATP synthase, making it function as an ATP generating rotor turned by Na+ re-entering the cell. This would generate ATP as fast as the proton gradient could produce a Na+ gradient. Very fast.
Possibly the first priority after the generation of a Na+/H+ antiporter would be to work out how to stop it. Here's how:
Proto-Ech is essentially sidelined as a source of high energy compounds by the novel Na+ energetics system. Mutations in NuoH or the NiFe hydrogenase would no longer be fatal, so there is the scope for the now redundant proto-Ech to be converted to a metabolic sensor and switch.
Excess reduced ferredoxin indicates a surplus of energy supply, almost certainly driven by over zealous antiporting. If this excess reduced ferredoxin is allowed to drive through proto-Ech, pushing electrons towards the FeNi centre and beyond to reduce protons to H2, this sign of excess could easily be adapted to produce a conformational change in NuoH.
NuoL is stuck on the side of NuoH. Changes in the shape of NuoH produce changes in both shape and function in NuoL. All that is needed is for excess reduced ferredoxin to produce a conformation change in NuoH and it could shut off the antiporting in NuoL. Having the antiporter (NuoL) glued to the side of NuoH allows the switch to function effectively.
Summary so far: Excess reduced ferredoxin stops/reduces antiporting and so controls ATP over-synthesis derived from exuberant Na+ energetics.
So here is the next question: What happens when the geothermal proton gradient fails? For a start, the free lunch from antiporting disappears. We still have the back up of ferredoxin energetics from electron bifurcation but nothing to compare to the glut of ATP from the now diminishing Na+ energetics.
What is needed is some way of pumping Na+ out of the cell which could replace the simple and exceedingly easy antiporting system. I've already got reduced ferredoxin acting through redundant proto-Ech as a brake on antiporting, as part of a control system on Na+ energetics. If we were to generate a change with allowed us to "over-apply" the ferredoxin brake we could, plausibly, go so far as to do more than simply stopping Na+ entering the cell, we might actually start to reverse Na+ ion flow and pump it outwards. Na+ pumping by using the energy derived from reduced ferredoxin.
Proto-Ech is now running in reverse and pumping Na+ ions. It is conserving energy as a Na+ gradient and so is now a true Ech, there no longer anything "proto" about it. It's reversible. Na+ energetics are restored and the core power supply to the cell is electron bifurcation supplying power to drive Na+ energetics.
That's how it stays until cytochromes come along.
Peter
Once people start looking at modern membrane complexes there is no need for such conventions and figures are usually produced the other way up. So when we want to look at modern Ech and derivatives I think I have to turn my proto-Ech doodle upside down like this:
I have, very naughtily, butchered a cartoon of a modern Ech (out of the lovely paper from Efremov and Sazanov) to produce this image of my concept of proto-Ech. As in the doodle, this must use a geothermal proton (not Na+) gradient and does not look to be reversible:
Note that I've altered the black arrows to match my red arrows, showing the generation of reduced ferredoxin from oxidised ferredoxin and the consumption of (primordial) H2 under the influence of oceanic proton flow through the brown membrane protein labelled EchB but which I will refer to from here onwards as NuoH, or a homologue there-of. Their original image of a modern Ech on which I based my proto-Ech is this one:
Again, for various reasons, I will refer to the blue subunit EchA as NuoL. This modern Ech is energy converting, it pumps protons through the blue anti porter homologous to NuoL. All of the arrows are now in their modern pumping direction. The pump is powered by reduced ferredoxin (from elsewhere in the cell) and produces hydrogen as waste. Typical bacteria using this Ech might be E coli, to produce flammable flatus. The source of reduced ferredoxin is any catabolic process. Fermenting starch in the anaerobic colon has predictable results in this department.
An understanding of the initial role for this antiporter is crucial to any logical approach to bioenergetic evolution. Equally interesting are the adaptions of subunit EchB (homologous to NuoH) to changing the activity of NuoL from antiporting to Na+ pumping.
We can now look at a NuoL type antiporter embedded in a membrane without any power supply. It will consume membrane proton potential by allowing protons to move inwards and expel Na+ ions outwards in exchange, like this:
It can do this in a modern bacterium because there is a proton gradient, created by complex I (or any other energy converting complex/hydrogenase), i.e. protons are available to travel down a concentration gradient in to the cell, much as they might have done in primordial times. Na+ ions are forced out against a concentration gradient, in this case because this particular microbe lives in an extremely high Na+ environment. If you delete the Mrp genes for subunits MrpD and MrpA you cripple its antiporting ability. Engineering back in homologous proton translocators from a modern complex I restores the full antiporting ability.This tells us that the modern proton translocators of complex I are still antiporters but their antiporting is kept suppressed. I would suggest by NuoH. Note that the Mrp antiporting complex has no NuoH subunit.
NuoL does appear to be distantly related to NuoH, possibly by very ancient gene duplication. The conservation of form can be seen in these lovely images from Marrreiro et al. The gold ribbon is NuoH and the grey one is NuoL. The channel form is clearly visible. I consider NuoH to be homologous to the original proton ion channel from my proto-Ech doodle and its relative NuoL to be homologous to a prototypical Na+/H+ antiporting derivative.
I think that the initial gene duplication/modification would probably have produced an uncontrolled antiporter which would have produced a dramatic fall in intracellular Na+ concentration. This activated ATP synthase, making it function as an ATP generating rotor turned by Na+ re-entering the cell. This would generate ATP as fast as the proton gradient could produce a Na+ gradient. Very fast.
Possibly the first priority after the generation of a Na+/H+ antiporter would be to work out how to stop it. Here's how:
Proto-Ech is essentially sidelined as a source of high energy compounds by the novel Na+ energetics system. Mutations in NuoH or the NiFe hydrogenase would no longer be fatal, so there is the scope for the now redundant proto-Ech to be converted to a metabolic sensor and switch.
Excess reduced ferredoxin indicates a surplus of energy supply, almost certainly driven by over zealous antiporting. If this excess reduced ferredoxin is allowed to drive through proto-Ech, pushing electrons towards the FeNi centre and beyond to reduce protons to H2, this sign of excess could easily be adapted to produce a conformational change in NuoH.
NuoL is stuck on the side of NuoH. Changes in the shape of NuoH produce changes in both shape and function in NuoL. All that is needed is for excess reduced ferredoxin to produce a conformation change in NuoH and it could shut off the antiporting in NuoL. Having the antiporter (NuoL) glued to the side of NuoH allows the switch to function effectively.
Summary so far: Excess reduced ferredoxin stops/reduces antiporting and so controls ATP over-synthesis derived from exuberant Na+ energetics.
So here is the next question: What happens when the geothermal proton gradient fails? For a start, the free lunch from antiporting disappears. We still have the back up of ferredoxin energetics from electron bifurcation but nothing to compare to the glut of ATP from the now diminishing Na+ energetics.
What is needed is some way of pumping Na+ out of the cell which could replace the simple and exceedingly easy antiporting system. I've already got reduced ferredoxin acting through redundant proto-Ech as a brake on antiporting, as part of a control system on Na+ energetics. If we were to generate a change with allowed us to "over-apply" the ferredoxin brake we could, plausibly, go so far as to do more than simply stopping Na+ entering the cell, we might actually start to reverse Na+ ion flow and pump it outwards. Na+ pumping by using the energy derived from reduced ferredoxin.
Proto-Ech is now running in reverse and pumping Na+ ions. It is conserving energy as a Na+ gradient and so is now a true Ech, there no longer anything "proto" about it. It's reversible. Na+ energetics are restored and the core power supply to the cell is electron bifurcation supplying power to drive Na+ energetics.
That's how it stays until cytochromes come along.
Peter
Subscribe to:
Posts (Atom)