This is a study which Raphi threw out on Twitter several weeks ago. It can take me personally this long to understand some studies. It's interesting as it clearly shows that weight loss over 12 weeks causes a deterioration in insulin sensitivity in some people:
They started with 52 obese people and ended up with 38 who completed this part of the study. On average insulin resistance dropped with weight loss.
On average, that is. It did for some. For others it didn't. Yes, in nine of the group of 38 subjects, insulin sensitivity deteriorated with weight loss. As the authors comment:
"Thus, it may seem paradoxical that some of the changes observed in the present study during weight loss prevented an increase in insulin sensitivity."
"Thus, it may seem paradoxical that some of the changes observed in the present study during weight loss prevented an increase in insulin sensitivity."
I love a paradox.
What is going on?
Here are the metrics from Table 2
Here are the metrics from Table 2
The paradox comes from the M values.
These are the clamp values. Insulin was infused to maintain a plasma level of 80microIU/ml and hypoglycaemia was avoided by infusing glucose to maintain a plasma level of 5mmol/l. If you do this for 2h you can assume some sort of steady state is achieved and the M value is the rate of infusion of glucose needed to counteract insulin's hypoglycaemic effect over the final 40 minutes of the clamp. The higher the M value the better insulin is working and the less insulin resistant your subject is. This is the gold standard, HOMA-IR is the poor relative (though much easier to estimate and wouldn't have shown any paradox in this study).
So in nine subjects the M value deteriorated with weight loss.
The subjects were divided on the basis of the direction of this change in M score resulting from weight loss.
To cut a long story short the group which deteriorated their M score were obese but had normal insulin sensitivity before weight loss. The so-called metabolically healthy obese. The subjects whose M score actually improved were insulin resistant before weight loss.
From a previous paper by the same group the M score in a group of lean control subjects (not described here but part of the overall study) was 9.77mg/kg ffm/min and here, for the obese but insulin sensitive group M, it was 9.17mg/kg ffm/min, ie no difference. In the obese but insulin resistant subjects pre weight loss M values averaged 5.63mg/kg ffm/min, ie low, p<0.05.
The first question is: why should metabolically healthy, insulin sensitive obese people deteriorate their insulin sensitivity score as a result of weight loss?
That's the simple one. They're losing weight and have not stopped at the time of the hyperinsulinaemic clamp. Their diet is providing completely inadequate calories to maintain stable weight so they are losing some weight, mostly from fat. The process of weight loss involves releasing FFAs from adipocytes with an associated rise in plasma FFAs and subsequent oxidation of those FFAs. I did a ball park rough calculation and someone of my height (but obese) would have been on 1400kcal per day, ie they would be hungry and oxidising body fat to make it up to the well over over 2000kcal I'm considered to require to run my metabolism.
Aside: Obviously from the Protons perspective oxidising fatty acids will generate ROS irrespective of mitochondrial delta psi and the cell will resist insulin's signal in proportion to this fatty acid oxidation induced ROS signal. It is apparent that this cannot be suppressed by an hyperinsulinaemic clamp for two hours, see below. End aside.
There is a study which actually measured fat oxidation under an hyperinsulinaemic clamp under conditions of active weight loss. It's this one:
Prolonged Fasting Identifies Skeletal Muscle Mitochondrial Dysfunction as Consequence Rather Than Cause of Human Insulin Resistance
which combines a Dutch version of the M score with indirect calorimetry during the steady state conditions of the clamp. Neat hey? This time weight loss was not from an hypocaloric diet, it was from a zero caloric diet, ie extended fasting, which sources calories from a minimal carbohydrate, minimal protein, very high fat source, ie the subject's own body catabolism. Just for 60 hours. But by the end nearly 100% of calories from are fat oxidation.
Prolonged Fasting Identifies Skeletal Muscle Mitochondrial Dysfunction as Consequence Rather Than Cause of Human Insulin Resistance
which combines a Dutch version of the M score with indirect calorimetry during the steady state conditions of the clamp. Neat hey? This time weight loss was not from an hypocaloric diet, it was from a zero caloric diet, ie extended fasting, which sources calories from a minimal carbohydrate, minimal protein, very high fat source, ie the subject's own body catabolism. Just for 60 hours. But by the end nearly 100% of calories from are fat oxidation.
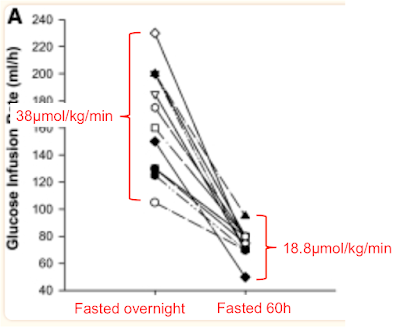
The clamp data come from Fig 2, the rates of disposal of glucose in red come from Table 2 and I've clarified the labels to point out that "Fed" means "Overnight fasted", not post prandial:
The glucose disposal units don't include fat free mass as this doesn't noticeably change in 60 hours of fasting so this means the values can't be directly compared to M values. We can see that glucose disposal halves under hyperinsulinaemic clamp study conditions when 60 hours of fasting necessitates metabolism to be run on fatty acids.
Proof that fatty acid oxidation continues under hyperinsulinamia comes from measurements in a respiratory chamber while the clamp was on-going. From the bottom of Table 2:
After an overnight fast, on a balanced diet, lipid oxidation is 1.2μmol/kg/min and drops to 0.3μmol/kg/min under clamp. During body fat loss for 60 hours the clamp can only suppress lipid oxidation to 1.52μmol/kg/min, ie still higher than under basal conditions before the 60 hour fast. Oxidising lipids intrinsically generates mitochondrial ROS to limit ingress of excess calories by limiting insulin facilitated signalling. Even at clamp levels of insulin exposure.
So weight loss causes insulin resistance because it intrinsically involves fatty acid oxidation. Stopping the weight loss using a period of adequate feeding would allow the weight loss induced insulin resistance to fade away. The only "paradox" is that the changes which occur in the provision and oxidation of fatty acids cannot be immediately reversed by the external application of 80μIU/ml of insulin, at least not within two or three hours.
It's also worth mentioning that this insulin resistance during weight loss is utterly normal and pro-survival from an evolutionary perspective.
Okay. Much more interesting are the patients who do improve their insulin sensitivity with weight loss. Fasting FFAs before weight loss are around 0.71mmol/l, higher than the value of 0.52mmol/l found in the slim control group in the previous section of the study.
This group has metabolic syndrome, ie they do not have the ability to suppress lipolysis using insulin because enough of their adipocytes are "leaking" FFAs via basal lipolysis and so are exposing their peripheral tissues to an on-going supply of FFAs, which are being oxidised continuously whether insulin is present or not. When insulin is present then this unstoppable supply of FFAs necessitates enough resistance to insulin's signal to balance caloric ingress. Glucose disposal under hyperinsulinaemic clamp (M value) stays low at 5.63mg/kg ffm/min because fatty acids are being oxidised, so glucose is significantly excluded. We don't know the FFA concentration under clamp or fatty acid oxidation in this study but my view is this is what is happening.
With weight loss adipocytes shrink. Two things happen. FFA levels rise because weight loss requires fat release. And, as adipocytes shrink, they decrease their size-determined basal lipolysis rate and so restore insulin's ability to suppress FFA release. These opposing factors, in this case, balance out in a trivially raised level of fasting FFAs to 0.76mmol/l, but the release of these FFAs can be suppressed by insulin, so M rises from 5.63mg/kg ffm/min to a respectable 8.55mg/kg ffm/min under the hyperinsulinaemic clamp after weight loss.
Fat oxidation obligates a degree of resistance to insulin's signal to maintain physiological caloric ingress. Protons also suggests some fatty acids do this better than others.
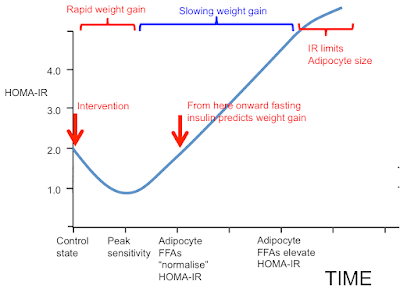
I was going to stop here but I may as well throw this in. People may recall this graph:
Now we can get rid of most of the labelling and insert the concepts discussed in this post. These arrows represent what I consider the situation to be under weight stability, so the situation is not complicated by the level of insulin resistance superimposed by weight loss.
It's easiest to think of the arrows representing the study subjects before 12 weeks on a severely hypocaloric diet. During weight loss the metabolically healthy group will temporarily move their location to the right due to increased fatty acid oxidation resisting insulin. Those with metabolic syndrome will try to do the same but this is offset by shrinking adipocytes decreasing their basal lipolysis so allowing insulin to work and actually improve the M score overall.
I could have stopped here too but I still have a final arrow to add. This comes from Arne Astrup's lab in Denmark. There are no clamp data but under weight stability the M score and HOMA-IR would correlate perfectly well, assuming everyone was on a similar mixed diet. Astrup looked at formerly obese (FO) women who had maintained, through sheer willpower, a normal body weight using conventional dieting. Rare as hen's teeth, but they do exist.
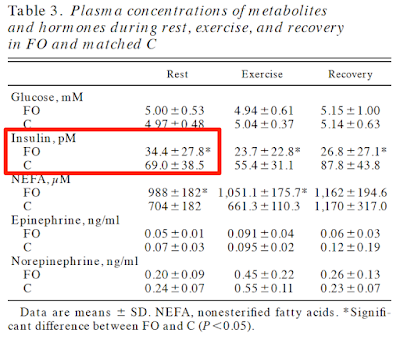
Fat metabolism in formerly obese women
Fat metabolism in formerly obese women
No clamp data but just look at the insulin and glucose values. C is control, FO is formerly obese. Formerly obese ladies are very, very insulin sensitive. Pathologically so:
If we wanted to add these ladies to the graph they would be here, at the blue arrow:
There is a body of opinion on t'internet that holds that insulin resistance causes obesity. They have some explaining to do.
Now I really will call it a day.
Peter