This is just a longevity study but it provides insight following on from my last post.
Let's just recap:
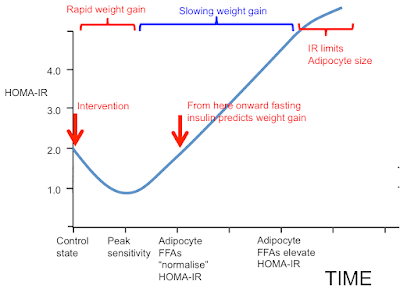
If we start in "control" state and switch to an insulin sensitising diet we have an initial sequestration of fat in to adipocytes and rapid weight gain, red bracket. As adipocytes distend they increase their basal lipolysis, which cannot be suppressed by insulin, and both fasting insulin and post prandial insulin levels must rise. If we fail to observe the initial acute insulin sensitivity phase and limit our observations to the rising insulin resistance phase (blue bracket) we could easily observe that elevated insulin levels are *associated* with future weight gain and assume, incorrectly, that there is causality.
On to the study.
The fat in the control diet is 15% soybean oil, so approx 7% of energy intake as LA. This is not a particularly low LA diet and, as you would expect, fasting insulin levels slowly rise throughout life.
All of the following graphs are extracted from Figure 2 section J, which looks like this
 |
giving this as the control insulin levels:
The high fat (aka insulin sensitising, aka increased LA) diet substituted lard for some of the carbohydrate and was fed ad lib. We don't know how much LA was in the lard, but probably at least 10% of the lard calories were LA. We miss the insulin sensitising hypoinsulinaemic phase as it's long gone by 28 weeks of age:
If we take this graph at face value, at any given age the insulin value is higher in the rats which are fattest, so the logical assumption is that this is causal. QED.
EDIT: I have used my imagination to butcher the above graph back to week eight. Accept or reject at your own discretion!
END EDIT.
But my contention is that these insulin levels are only elevated as a consequence of adipocyte size, ie the hyperinsulinaemia is a secondary phenomenon and could be eliminated by suppressing basal lipolysis, usually using good old acipimox. It works.
But acipimox cannot maintain suppressed lipolysis from distended adipocytes for more than a few hours.
Undoubtedly the most effective way of limiting basal lipolysis is to limit absolute adipocyte size. If we could maintain the adipocytes of the rats fed the insulin sensitising diet at the same size as those of the control fed rats there would be no "masking" effect of elevated basal lipolysis to hide the on going life long insulin sensitising effect of the obesogenic diet.
So they pair fed, by calories, exactly the same "calories" to a third group of rats so that they received exactly the same calories of insulin sensitising food as the chow fed mice eating ad lib.
These rats dis not become fat (I know, I know, heavy calories vs light calories, forgive me) and their weights exactly matched those of the control rats. So now we have rats with normal levels of basal lipolysis for age but no excess through eliminating "over eating" (stop sniggering) the amount of food which hunger, driven by excess insulin sensitivity, demanded in the "green-line" rats.
So the red line is the true insulin sensitivity of rats fed the obesogenic diet when not allowed to superimpose the increased basal lipolysis generated by becoming fat per se:
I hope this post complements the previous post and makes it completely clear that an insulin sensitising diet causes rapid sequestration of fat in to adipocytes. This lost fat will a) make you hungry, b) distend your adipocytes, c) increase their basal lipolysis and d) this latter will cause systemic insulin resistance.
This is a very basic and very simple to understand concept.
Aside: d) is core to limiting caloric ingress to cells when FFAs are available in the presence of insulin/glucose, which should not occur. You know, as in
which is a fundamental paper for understanding hyperinsulinaemia. Ignore at your peril. End aside.
What happens to make adipocytes insulin resistant (a late change in the words of Bill Lagakos, and I agree) is much, much trickier and then we're slipping in to Nick Lane territory.
Peter





17 comments:
So, how to reverse this fate?
Saturated fat.
P
@TS
Paradoxically, this can be solved by slow and shallow breathing, retaining CO2 in the lungs. This oxygenates the tissues and switches back from hypoxia to oxidative phosphorylation.
https://mct4health.blogspot.com/2023/05/carbon-dioxide-against-lack-of-oxygen.html
@Peter
Saturated fats will be desaturated immediately, so it won't help much. Medium length cannot be desaturated, so hopefully they will help better.
Jaromir
"So now we have rats with normal levels of basal lipolysis for age but no excess through eliminating "over eating" (stop sniggering) the amount of food which hunger, driven by excess insulin sensitivity, demanded in the "green-line" rats." - I wasn't sniggering. Too sad. Poor rats. And poor humans for that matter :/
"Undoubtedly the most effective way of limiting basal lipolysis is to limit absolute adipocyte size. If we could maintain the adipocytes of the rats fed the insulin sensitising diet at the same size as those of the control fed rats there would be no "masking" effect of elevated basal lipolysis to hide the on going life long insulin sensitising effect of the obesogenic diet."
- there are populations (Asians) in whom fat cells tend to hypertrophy as opposed to hyperplasia - and hopefully the PC brigade doesn't hang out here to come after me for saying this :eyeroll: - so they can't gain that much weight, and suffer from horrible consequences at much lower bmi's. So they won't be able to limit basal lipolysis at all, right?
Yes, like a mild version of Berardinelli-Seip syndrome...
BTW the hungry rats lived longest (median lifespan). I recall a CRON eater once speculating that if, at 50 years old, he lived to be 150, he had a century of hunger ahead of him.
Peter
TS: So, how to reverse this fate?
Peter: Saturated fat.
I would put this slightly differently although it ends up in a similar place. If you are in a state of insulin resistance plus hyperglycaemia the first most important thing to do is to stop consuming any carbohydrates. Perhaps the worst thing you could do would be to increase the amount of fats/oils without eliminaing sugar and starch. There also needs to be a marginal phase, a transition to the new approach, either by fasting for a couple of days or with the use pharmaceuticals such as metformin because hyperglycaemia has some 'inertia'. Blood sugar stays high for a long time, even without carbs, if you start from a seriously insulin resistant state and you need to reduce the amounts of stored fats in adipocytes. Excercise, of course, is not a bad thing either.
Jaromir, as I understand it if you don't have significant unsaturates in your diet fat storage is mostly via saturated palmitic and stearic triglycerides. It is the preferred form. Hence 'animal' fat. Humans are animals so ...
Oh, and to add to first para above, eliminate polyunsaturated fat intake. No more seed oils. But I think the carbs have priority.
@Pass
As I see it, saturated fats would work if they were burned. But if you are in fat saving/storing mode, they will be blocked by malonyl-CoA, desaturation is overactivated and they are storedas unsaturated, not burned. If you really want to burn them, you must suppress lipolysis, restore glutathion recycling and make some CO2 to get O2 for cell respiration. You can do it by MCT, it clean up FFA, promote making of dicarboxylic acids and succinate to direct all fats to peroxisomes and allow PDH complex for carbs metabolism and with succinate from peroxisomes it makes enough H2O2 - NADP+ and CO2 on IDH2. But you have to restore glutathion somehow, e.g by glycine. This is the most important part for restoring normal fat metabolism.
Ketones also make some CO2 from acetate to allow respiration, I suppose, but I would not call it normal fat metabolism.
Peter - never heard of Berardinelli-Seip before, horrible and fascinating.
(My family are half MD's, half engineers. I spent my childhood reading through mum's case description books and atlases and whatnot. It's so incredibly interesting and sad all at once). Sent me down an interesting rabbit hole!
"I recall a CRON eater once speculating that if, at 50 years old, he lived to be 150, he had a century of hunger ahead of him." - LOL, some people would consider 100 years of starvation a cruel and unusual punishment, but what do I know :D Personally - I think I'll pass on that :P
Mct4health:
Something doesnt quite add up there!
Eg
"Palmitic acid (16:0, PA) is the most common saturated fatty acid found in the human body and can be provided in the diet or synthesized endogenously from other fatty acids, carbohydrates and amino acids. PA represents 20–30% of total fatty acids (FA) in membrane phospholipids (PL), and adipose triacylglycerols (TAG)"
From
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5682332/
@Pass
Yes PA is stored, but not burned as much as it should be. There is strong tendency to lower stearic acid in fat storage over time. PA will not be converted to SA but to oleic acid and this continue to support fat storage in vicious cycle. Brad Marshall about it in https://youtu.be/YaM0KJzaZqU
@Peter
The first phase of insulin sensitivity could be also heat producing without fat making. Only after glutathion depletion and acetylation of respiration enzymes it converts to fat making and insulin resistant, I think.
If PUFA by DECR enzyme disconnect H2O2 from CO2 production, then H2O2 is temporarily not needed and excessive respiration (NADP+ to IDH2) and heat production could be out of control.
https://mct4health.blogspot.com/2023/06/omega-6-polyunsaturated-fats-disable.html
@Pass
One more Brad Marshall:
Fat Metabolism in Context: PPAR Alpha and NAD+
https://youtu.be/MxUfxMnPZkk
Palmitic acid goes through the processes of being liberated from triglycerides and on through beta oxidation straightforwardly. Otoh unsaturated bonds stop beta oxidation in its tracks and require the action of various extra enzymatic processes to allow BetaOx to continue, therefore less efficiently, with different enzymes and processes depending on exactly where the double bond occurs.
Eg H. Schulz, in Encyclopedia of Biological Chemistry (Second Edition), 2013
"Unsaturated and polyunsaturated fatty acids also are degraded by β-oxidation. However, additional reactions are required to metabolize pre-existing double bonds that would otherwise interfere with the complete β-oxidation of unsaturated fatty acids."
Pass—"Otoh unsaturated bonds stop beta oxidation in its tracks and require the action of various extra enzymatic processes to allow BetaOx to continue, therefore less efficiently"
Thank you, that's the factoid I've been searching for for a long time—that double bonds halt BetaOx.
CN, the current wikipedia item on beta oxidation is quite good. It works through the various possibilities: odd/even, sat vs w3, w9, and parts of the energy budget.
https://en.m.wikipedia.org/wiki/Beta_oxidation
My take is the terminology tends to mislead the conversation. We don't call opiate-tolerance opiate-resistance. Why do we call insulin-tolerance insulin-resistance? Could it be that looking at average insulin levels might be more useful than A1C or cholesterol tests?
Take most any drug and your metabolic system will try to compensate with tolerance. Including injections of insulin.
Insulin-tolerance begs the question of what is causing chronic exposure to high levels of insulin? ( some food choices are obvious, but others less so - artificial sugars - that life hack that went wrong - and untested food additives - many are antibiotics - in the GRAS category. (redefine the term! GRAS(Guesses Represented As Science) and it all makes sense.))).
And like other drugs - insulin is not specific to blood glucose - In the long list of effects some low-carb folks know it causes the sodium retention that is blamed on salt in the diet.
One other effect of chronically elevated insulin, I think is involved in CVD. Decreased autophagy - decreased level of degradation of damaged organelles. Postprandial levels inhibit autophagy completely.
If you go without food for a while, autophagy returns - and we enter a catabolic state. I think that narrowing of arteries starts with a blood-clot - that then gets covered over with new intima - the remnants of the clot, (often in visible layers) are what gets call plaque. The narratives your MDs were/are taught are simply wrong).
If one stops eating by say 6PM - there are now hours of the day where your body can resorb blood clots - and keep your arteries from narrowing.
For people that are quite obese - even stopping eating for a while won't lower insulin.
From what I can see - the T2D pandemic is the distal cause of so many deaths, (via cancer/CVD/kidney failure /etc/etc..) that it is THE health crises of our time.
,.,.
wondering OT..
The other day, I was over for dinner - tacos - they had a bag of shredded cheese.. I looked at the ingredients - Natamycin. Called by some a preservative - in fact it is an antibiotic - dosed here at a level that required it to be on the label.
The -mycin suffix like in erythromycin, streptomycin, terramycin etc.. gives it away. So do you want 500mg of antibiotic with your cheese?
It is listed as GRAS.. but we know that antibiotic trigger weight gain in livestock - used for just that purpose.
But don't be fooled - most of the food additives in packaged foods - with ridiculous shelf life have GRAS additives.
Calling untested additives GRAS makes money flow.
Hi karl,
Cross posted from another thread but the gist of it is related to the first part of your comment.
Personally I think we are using the one term to describe a number of quite different phenomena. The simplest might be the inability to suppress lipolysis in adipocytes, ie basal lipolysis, ie the proportion of large adipocytes in the body/visceral fat, ie an euglycaemic hyperinsulinaemic clamp. That is not the same as CoQ depletion by a statin drug or glutatiolation of complex I to limit ROS generation.
Also the insulin resistance of fasting will disappear over 30 minutes if you infuse glucose/insulin, so time course matters. Ditto pre/post prandial.
I have other examples but you get the flavour.
Will cross post this to another comment thread as karl made a related point.
Peter
Post a Comment