This is another non-referenced, thinking out loud post which is the precursor to more normal technical posts. Here we go.
The whole underpinning of the Protons concept is that inadequate generation of ROS secondary to the presence of double bonds in fatty acid fuels causes pathological insulin sensitivity.
Too few ROS.
This post is about how generating too few ROS in mitochondria generates excess ROS in those said mitochondria and what physiology does about this.
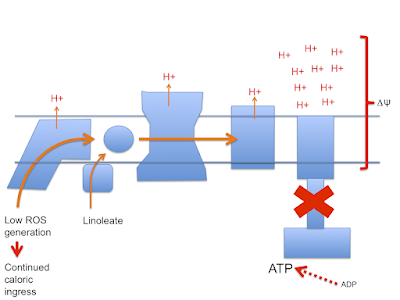
Here's the stripped down doodle of the electron transport chain I'm going to use
I've left out complex II, cytochrome C etc to keep it very simple. It's not complete, it's a minimal mental "model". You have been warned.
Next is the normal electron flow from NADH and FADH2 to oxygen:
Electrons passing through complexes I, III and IV pump protons out of the mitochondria to produce an electrical and pH difference between inside and out, the mitochondrial membrane potential, delta psi
and of course delta psi is used to generate ATP by ATP synthase, much as a rotating water mill uses hydrostatic pressure to generate usable energy.
One crucial necessity to allow ATP synthase to function is a supply of ADP. If all of a cell's supply of phosphorylated adenine is in the form of ATP there is minimal ADP as substrate for ATP-synthase to act on, so delta psi will become larger as pumped protons accumulate on the outside of the mitochondrial membrane.

If ATP predominates the cell is replete and the correct response is to limit further caloric ingress. As ETFdh tries to transfer electrons on to the CoQ couple and subsequently to complexes III and IV it becomes progressively harder to pump protons against a rising delta psi. At some point it becomes easier to divert electrons from ETFdh through complex I as reverse electron transport (RET) which generates a very specific, localised ROS signal which is designed to limit insulin signalling and be quenched using superoxide dismutase without doing damage. Saturated fats produce this signal very well because their lack of double bonds maximises the input of FADH2 to facilitate RET:
In this mental model palmitate limits caloric ingress, stops excessive proton pumping and maintains delta psi within physiological limits.
Next we have the situation under linoleic acid oxidation. Here there is a reduced input of FADH2 from ETFdh so it is more difficult to generate the RET needed to limit caloric ingress when ATP is replete and ATP-synthase is no longer consuming delta psi. Protons continue to be pumped and delta psi rises to supra-physiological levels
As delta psi rises the ability to generate RET through complex I also rises until eventually even the relatively low FADH2 input from linoleate can produce RET. However this high delta psi will also allow the generation of ROS at multiple sites in the ETC in addition to that at complex I. Complex IV seems to be a minimal site for this but complex III will produce ROS from sites facing both the mitochondrial matrix and the cytoplasm while complex I appears to only generate on the matrix side, probably from multiple sites under very high delta psi. ETDdh (and mtG-3-Pdh and complex II) can also generate ROS under high delta psi conditions:
This is both good and bad.
Good because it finally hits the signalling pathways needed to limit caloric ingress. Bad because it hits lots of other components of the cell structure in addition.
Summary: consuming linoleic acid will cause your mitochondria to explode.
Except that's preposterous, they don't.
The simplest protective measure is the diversion of calories within the cell in to storage. Those calories are only present in the cell secondary to excess insulin signalling. Because diversion to storage is a classical function of insulin signalling, this will drive obesity whilst also providing some protection from pathological ROS generation.
Additional protection comes from uncoupling.
The core mechanism for the generation of excessive ROS under unmitigated LA oxidation is high delta psi.
Uncoupling lowers delta psi. Doing this is all that is necessary to protect against pathological ROS generation.
But wait.
If linoleic acid allows excess caloric ingress due to a deficit in physiological ROS generation, surely non-specific suppression of ROS generation should increase caloric ingress, increase delta psi to overcome the degree of uncoupling present and re establish pathological ROS generation? Alternatively might uncoupling go on to allow even more excess calorie storage?
Neither happens.
I'll run through some of the papers I've been looking at over the last few months and post about them next.
Peter