People may have noticed that I'm quite interested in basal lipolysis, adipocyte size and metabolic syndrome. That is correct.
What happens if you delete adipose triglyceride lipase (ATGL) so you can't have basal lipolysis? This paper gives some answers as to what happens to mice with ATGL knockout
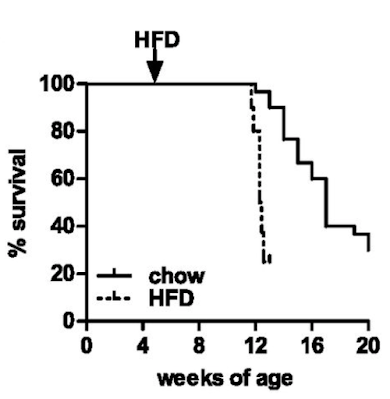
Hypophagia and metabolic adaptations in mice with defective ATGL-mediated lipolysis cause resistance to HFD-induced obesityWell, they die. Not unexpected. Here are the survival curves, one group of ATGL knockouts fed chow and another fed an "high fat diet" based on a modified D12492 (mostly extra sucrose with the lard)
If you consider which organ runs (in health) almost exclusively on fatty acid oxidation it will come as no surprise that the mice die of dilated cardiomyopathy secondary to lipid accumulation and mitochondrial failure. Sooner and more rapidly on the high fat diet.
You can get round this problem by engineering the ATGL gene just in to cardiac myocytes. Then the animals live long enough to allow you to study the effects of ATGL deficiency in the absence of a dead myocardium. The whole of the paper, other than Figure S1, uses mice with this protected myocardium (denoted cTg). WT/cTg denotes normal ATGL throughout their body plus extra myocardial ATGL (phenotypically normal) or AKO/cTg without ATGL everywhere other than their myocardium. So ignore the cTg label part, its WT vs AKO re adipocytes throughout the paper
So here's the paradox.
On chow WT mice carry approximately 2g of fat and AKO mice carry 5g of fat, much as the histology suggests and as you might expect. Figure 1 summarises the top left and top right groups of mice:
Things get more interesting when we compare the high fat fed WT mice with the high fat fed AKO mice, thats the bottom left and bottom right. Both groups have increased fat mass but the AKO mice are less obese than the WT mice. Like this
Note that all of the vertical scales are different. But there we have it, knocking out ATGL long term blunts the obesity induced by D12492, somewhat. The effect kicks in slowly but is well established by the end of the study at 22 weeks (solid black squares are AKO)
The paper goes in to some detail about PPAR-γ2 suppression in AKO mice which can be reverse by the diabetes PPAR agonist rosiglitazone.
Which ultimately translates as the adipocytes adapt to being unable to offload triglycerides by suppressing every aspect of lipid uptake and storage that they can.
"... the expression of genes involved in lipogenesis and fat storage such as PPAR-γ2 (−95%), C/EBPα (−30%), and SREBP1c (−78%) were significantly lower in gWAT from HFD-fed AKO/cTg mice than from WT/cTg."
The AKO mouse adipocytes, which cannot off-load lipid, compensate by progressively rejecting lipid ingress.
Does this make the adipocytes insulin resistant? Or the mice insulin resistant?
They didn't look at this at the adipocyte level and the interactions are too complex to guess how adipocytes might respond to physiological or pharmacological exposure to insulin.
What we do know is that the AKO mice fed a high fat diet are still very insulin sensitive at the whole body level despite their adipocytes eventually down regulating all aspects of lipid accumulation. Here's the intra-peritoneal glucose tolerance test result. All of the following results are high fat diet based.
If you can read Table S1 in the original paper (too faint to reproduce here) you can see that fasting insulin in the AKO mice is 0.1ng/ml vs WT at 1.0ng/ml. Fasting glucose is also low at 164mg/dl in the AKO mice vs 212mg/dl in WT. Sorry for all the Noddy units. HOMA-IR score would be very, very low for AKO mice.
It doesn't matter what size the adipocytes of an AKO mouse are, they are not going to perform basal lipolysis. Under fasting conditions in normal mice FFAs go up as augmented lipolysis frees FFAs and there is little insulin to limit further lipolysis and FFA release. We need elevated FFAs under fasting.
In Table S1 again the WT mice have a fed FFA level of 0.72mM which rises to 0.93mM on a 4h fast, as it should do. In the AKO mice fed FFAs are 0.65mM and drop to 0.45mM on a 4h fast. They drop on fasting, so we can assume that the initial 0.65mM fed value is largely diet derived and so, with no food and no basal lipolysis, FFA levels have to fall.
Which they do.
My premise from Protons is that insulin resistance is an adaptive response to the delivery of FFAs. Under fasting this is ideal. In the presence of elevated glucose and insulin then the elevated FFAs from distended adipocytes cause caloric oversupply to the whole body and insulin resistance has to kick in to adapt. It is an antioxidant defence mechanism to limit ROS generation to physiological levels.
No ATGL -> perennialy low FFAs -> no need to resist insulin -> insulin sensitive
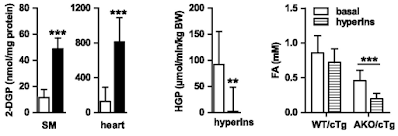
If we look at the hyperinsulinaemic clamp data we can see that both skeletal muscle (SM) and heart in AKO mice are really good at taking up 2-deoxyglucose. The liver, on a diet of 28% sucrose by weight, is also *very* insulin sensitive, with near total suppression of hepatic glucose production (HGP) during the clamp:
Of course the interesting bar chart is the right hand end one. The basal FFA levels are the ones cited above after a 4h fast. Hyperinsulinaemia with normoglycaemia lowers fasting FFAs a little, but without statistical significance, in WT mice obese from D12492. Doing the same in AKO mice produces a marked fall from low levels to even lower levels, probably somewhere around 0.2mM.
Clearly the excess of plasma free fatty acids, derived from elevated basal lipolysis and which necessitate insulin resistance ie trigger metabolic syndrome, is not present in the AKO mice. Whatever the size of their adipocyte lipid droplets there is no fatty acid release. My guess for the fall in FFAs is that residual post prandial FFAs are being allowed in to muscle and liver cells using CD36 translocated to the cell surface in parallel to GLUT4s in response to the clamp.
Asides before I finish: What does the term "hypophagia" in the title of the paper actually mean? It means that the mice are NOT HUNGRY. They eat ad lib until they are satiated. Because they have down regulated their ability to "sequester" calories in to adipocytes, they sense adequate calories are available earlier so stop eating earlier. They are not "hypophagic", their lack of hunger is manifest as eating less. They're not under-eating. They're eating exactly the correct amount of food to supply their metabolic needs. It doesn't matter that the food appears to be hedonistic, rewarding or addictive (stop sniggering) as it appears to be in the WT mice. Under exactly the same hedonistic/rewarding/addictive food environment (you really must stop sniggering, and so must I) as the WT mice the AKO mice are simply NOT HUNGRY. The brain is such a secondary organ compared to the adipocyte.
Another aside: Where does hepatic insulin resistance in fructose fed mice (like the WT here) come from? Look here
Yes. Fructose, if it gets as far as adipocytes, forces FFA release. This will use ATGL. These FFAs will end up in the liver and have to be repackaged as VLDLs to be returned to the adipocytes. If insulin sensitivity is pathologically high (ie linoleic acid exposure) those FFAs in the liver will be stored there giving fatty liver, NAFLD etc. The AKO mice will obviously catabolise fructose without any problem but will be incapable of fatty liver because they cannot transfer fatty acids out of adipocytes to get to hepatocytes. Hence the incredible ability to suppress hepatic glucose production during the clamp in AKO mice. See HGP in the above figure. Adipocyte AGTL is essential for NAFLD on an high fructose diet.
Okay, I'll shut up now. The role of ATGL in converting linoleic acid induced insulin sensitisation in to whole body insulin resistance and metabolic syndrome is central. This extends to NAFLD and ALD.
Physiology is comprehensible.
Peter
I was going to hit post but two more asides have presented themselves to my brain. Rosiglitazone more than eliminates the down regulation of PPAR-γ2 in AKO mice to give a slightly more obese mouse than the WT high fat fed mice. Does this restore insulin resistance too? Of course not, those bigger adipocytes still can't do lipolysis. The group must know this so they simply didn't run IPGTTs on the rosi-fat mice.
Also re adipocytes under clamp conditions. They looked at behaviour of skeletal muscle cells, heart cells and liver cells under hyperinsulinaemia. But not adipocytes. They know these adipocytes are resistant to insulin, so they didn't check. In a paper on AGTL and adipocyte function. They know this. I love glaring holes from carefully crafted methods sections where you can see the not-investigated leverages.
Oh, and at the time of this study they were clearly looking to find a drug to recreated the benefits of AGTL knockout on weight gain (for high PUFA, high sucrose fed humans). If they had found one I guess they would just have crossed their fingers that it wouldn't trigger dilated cardiomyopathy.
Oh, and another. High PUFA, high sucrose diets clearly do not trigger insulin resistance in the absence of pathologically distended adipocytes forcing elevated basal lipolysis and caloric overload induced high delta psi. The high linoleic acid only generates the 4-HNE to augment insulin resistance and ultimately shut down ETC function when caloric supply and delta psi is excessive. These AKO mice have access to obesogenic levels of PUFA and 21% atmospheric oxygen, yet essentially zero insulin resistance.
Now I really will stop.