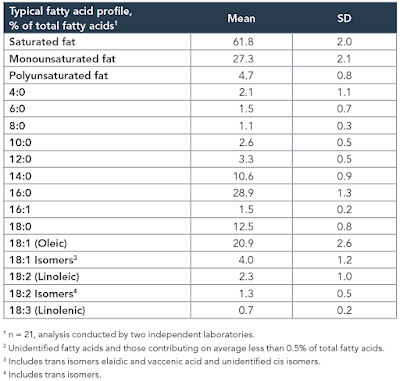
Mike Eades updated the current copy of the Arrow to include Alex Berenson's observation that the COVID-19 vaccine surveillance report Week 42 from the UK Health Security Agency (ie the UK Government, such as it is) details that the phenomenon of Original Antigenic Sin is clearly being demonstrated in the UK covid antibody data.
This concept is very simple and predicts that if you are exposed to a single antigen (here the spike protein derived from an mRNA vaccine) your immune system will prioritise a response to that single antigen in preference to other antigens when presented with a mixed antigen soup, as in the whole virus during a subsequent field infection.
So, in double vaccinated individuals you have preferential response to the spike protein over nucleocapsid protein as assessed by antibody titres. Page 23 if you want to have a look:
"recent observations from UK Health Security Agency
(UKHSA) surveillance data that N [nucleocapsid] antibody levels appear to be lower in individuals who acquire
infection following 2 doses of vaccination."
ie the vaccine screws your immune response to nucleocapsid.
Somewhat.
However the UKHSA only monitor anti-spike protein and anti-nucleocapsid antibodies because these allow us to distinguish between vaccine exposure and field infection. Obviously field infection triggers many more immune responses in addition to those against spike and nucleocapsid proteins, none of which need to be monitored to get this information.
As we vaccinate using the spike protein alone we will actively favour the survival of vaccine evading mutations. Boosters will speed this up.
So, are we all going to die?
I think not. UKHSA is monitoring antibodies. These are being surveyed in recovered patients.
"Recovered" is the word.
Ultimately triple (and greater, eventually) vaccinated people, so long as the vaccine is spike protein based, will eventually end up behaving as though they are unable to even "see" the spike protein, their anti-spike antibodies will be present but will do nothing. Spike evasion will have happened and the selection pressure will no longer be present. Lots of anti-spike antibodies, no interaction with the spike, no further selection pressure.
Vaccinated people will have to run on non-spike immune response, which will still be broad and still work. It may not be as effective as in the non vaccinated, because the immune system prioritises large amounts of useless spike response, but most people will still survive (unless they have chosen to be a poorly controlled diabetic, diagnosed or in-situ of course) as they are doing currently.
In some ways I can see some use for the vaccine and the idea of vaccine passports.
Aside: Of course using vaccine passports for anything, especially to pauperise and exclude the unvaccinated, will come with a sh!tload of human rights violations in addition to the health problems automatically generated by pauperisation per se. This is morally reprehensible and unforgivable. It's happening now if you live in the wrong country. Don't you love politicians? End aside.
At the start of the pandemic certain groups of people were thrown under the bus as regards covid. These are people who do actual work. Supermarket checkout cashiers, bus drivers, garbage collectors, postal workers, truck drivers, construction workers. Others, like myself, were given several months leave on 80% of salary with a big garden during some of the sunniest Spring weather I can recall. So we "let the virus rip" through people who actually do jobs ("essential workers") and paid loafers like myself ££££ of my children's and probably grandchildren's money to stay at home and "avoid" the virus. For a while.
Now the vaccine is here and the virus is in reality being allowed to rip through the rest of society, including the laptop classes. Clearly vaccine passports will actively concentrate vaccinated people in to crowded places and so maximise transmission. The UKHSE report cited above also reports vaccinated people are a lot better at getting infected compared to the unvaccinated, interestingly enough. Provided these people do survive (and most will) then they will end up with a ton of useless anti-spike "immunity" plus enough real immunity to other components of the virus to survive future exposure to that virus. We need this.
That should be enough.
Peter
PS I can live without the human rights violations which seem to be endemic at the moment. Or should I say epidemic or pandemic???