I usually start from the premise that insulin makes you fat. The most simplistic prediction from this is that eating carbohydrate raises insulin and this insulin is what makes you fat. Over the years I have looked at data from all sorts of places, particularly the extremes such as the Kempner Rice Diet and/or the Potato Diet, which clearly work and which appear to do so (to me!) via lowering the level of systemic insulin acting on adipocytes. In particular I consider that first pass hepatic insulin extraction has a huge effect on the systemic insulin level and the subsequent exposure of adipocytes to that insulin. The carbohydrate-insulin-model of obesity as set up by detractors is simplistic in the extreme.
This paper was published recently:
The carbohydrate-insulin model does not explain the impact of varying dietary macronutrients on body weight and adiposity of mice
Of all of the combinations tested the one which we are interested in is where protein was held constant at 10% of calories and carbohydrate was varied from 10% of calories up to 80% of calories. The remaining calories were made up of fat giving a pure carb vs fat comparison. At the end of the study period we have this graph where I have added in the percentages of calories from fat in red along the x axis:
If you draw a straight line through the data points your weight line slopes downwards as fat percentage drops, pretty well. Fat makes you fat:
Now, clearly, there is a missing data point. That is the bodyweight from a diet group with zero carbohydrate, 10% protein and 90% fat. This combination was not included in the study.
So all else from here forward in this post is now pure speculation.
Help is at hand for the missing data point in this paper
A high-fat, ketogenic diet induces a unique metabolic state in mice
Here we also have C57Bl/6 mice and in this case they were fed F3666 diet which looks like this:
"The proportion of calories deriving from different nutrients was as follows: ... KD:95% fat, 0% carbohydrate (0% sucrose), 5% protein"
This is not a perfect fit as the protein is about half that used by Speakman and there is (obviously) no sucrose, but it's the best anyone can do in the absence of the omitted group essential to complete the graph. The mice which were eventually put on to F3666 were initially made obese with a sucrose/fat combination before being put on to their ketogenic diet. Their weight dropped from approx 37g on the sucrose/fat diet to approx 27g on F3666, ie they ended up about 3g lower in total bodyweight than the control mice fed approx 10% of calories from fat in a carbohydrate based diet throughout. Here's the graph we all know from years ago. Open triangles show the drop in weight when F3666 was introduced at around day 80:
So what might a zero carb, fairly low protein group of C57Bl/6 mice look like? They might well end up slightly below the weight of Speakman's 80% carb group mice, (ie those eating closest to mouse chow), or they might end up slightly heavier due to the higher protein content limiting the total fat percentage which could be provided. I feel a compromise might be to use 35g, the same as Speakman's 80% carb group, the closest chow equivalent.
I've added the zero carb speculative data point to the blue line on the graph at 35g bodyweight which now looks like this:
and now we can curve fit the bodyweights like this:
Ah, that's better. Ketosis at the left, "carbosis" at the right. Nice.
I love rodent studies. You just have to understand that setting them up correctly is essential to obtaining the result you want. You also have to know what you want.
Peter
Wednesday, November 27, 2019
Monday, November 11, 2019
Protons (51) From peripheral cells to the brain
Hunger and satiety. Can these be modelled from a very simple energy availability concept?
This post is a minimally referenced ramble through how I see satiety working.
At the peripheral cellular level energy influx is controlled, to a large extent, via reactive oxygen species. These regulate insulin signalling which controls the translocation of GLUT4 and CD36 proteins to the cell surface and so facilitates the diffusion in to the cell of glucose and fatty acids respectively.
When a single cell has adequate calories it generates ROS, largely from the electron transport chain, which disable insulin signalling. This insulin "resistance" is there to limit excessive ingress of calories. ROS are the signal that no further calories are needed. At the most basic level cellular energy ingress is regulated by the core energy utilising apparatus of the cell. Excess substrate means excess ROS means shut down caloric ingress.
This is a self-contained cellular satiety signal of the most basic type. When the cell has fully adequate supplies of ATP, a high mitochondrial membrane voltage and a deeply reduced CoQ couple then ROS generation becomes almost inevitable.
It is a core, deep level, simple system. How it works is the subject of the Protons thread of posts, as is how it malfunctions.
I find it very, very difficult to envisage that the control of ingress of calories on a whole body basis (hunger/satiety) is not similarly integrated around an ROS generating system within dedicated neurons of the brain, with the hypothalamus being the most likely location.
I've had this as a persistent suspicion for a very long time but you can't get around to reading about everything at the same time. And I have to admit that neurological thinking about hunger and satiety has always struck me as a highly disreputable field. Insulin and satiety smell like cholesterol and the lipid hypothesis.
So yesterday I finally went and had a look for a reasonably recent review of ROS within the hypothalamus and this one came up pretty high in the PubMed listing
Impact of hypothalamic reactive oxygen species in the regulation of energy metabolism and food intake
This gives a flavour:
"Thus, it appears that NPY/AgRP neurons activation is mediated by a decrease in ROS levels while POMC neurons activation is driven by ROS (Andrews et al., 2008). Indeed, icv administration of ROS scavengers induces significantly lower c-Fos expression in POMC neurons and increases food intake during light cycle, observed via an increase of c-Fos expression in AgRP/NPY neurons (Diano et al., 2011). Similarly, addition of H2O2 depolarizes POMC neurons, increases the firing rate, and an icv injection of H2O2 causes significantly less feeding of mice after an overnight fast".
A lot of the work cited is not terribly well performed and no one has the Protons framework to slot their findings in to, but it's a start. What I am looking for is that these cell types do express GLUT proteins and CD36 proteins. I would expect them to be sampling arterial blood or CSF and integrating NADH and FADH2 inputs to their mitochondrial electron transport chain to "decide" whether there are adequate calories to consider that satiety or hunger might be the preferred descriptor of the body's current state.
Whether these cells express insulin receptors to facilitate ingress of substrate is something to be picked at. As I am completely biased against the concept that insulin is a satiety hormone, I would prefer this not to be the case but may be wrong. Time will tell. It looks logical to me that the brain would look at the nutrient levels present in excess of those being disposed of by peripheral insulin in to peripheral cells. As large numbers of peripheral cells become "full" under the influence of insulin, the brain should pick up the rising level of excess nutrients as the signal to call a halt on hunger. Doing this within the brain shouldn't need insulin, merely a set of relatively low affinity transporters to allow glucose and lipid uptake as insulin completes its peripheral function. Satiety should be picked up when enough cells have enough calories, whole body, that they no longer behave as a calorie "sump". The job of the brain is to pick up evidence from the nutrient levels that the sump is full and satiety can be declared.
For hunger, high affinity transporters would allow ROS to be generated easily and falling ROS would signal that energy availability was low.
These signals will come from the neural mitochondrial ETC generating ROS. The best ROS generating nutrients will be the most satiating. Saturated fats spring to mind if you follow the Protons thread.
Obviously there are whole load more hormones which can influence the generation of ROS within neurons. Physiology has applied layers and layers of signalling to maximise reproductive fitness. I have minimal interest in these "higher level" signals. They are there, they will modulate the core process I've been talking about but I see no way that they will do anything fundamentally different from or in opposition to the ROS system.
I'll give the rest of the review a bit of a read and see if it's worth posting about.
Peter
This post is a minimally referenced ramble through how I see satiety working.
At the peripheral cellular level energy influx is controlled, to a large extent, via reactive oxygen species. These regulate insulin signalling which controls the translocation of GLUT4 and CD36 proteins to the cell surface and so facilitates the diffusion in to the cell of glucose and fatty acids respectively.
When a single cell has adequate calories it generates ROS, largely from the electron transport chain, which disable insulin signalling. This insulin "resistance" is there to limit excessive ingress of calories. ROS are the signal that no further calories are needed. At the most basic level cellular energy ingress is regulated by the core energy utilising apparatus of the cell. Excess substrate means excess ROS means shut down caloric ingress.
This is a self-contained cellular satiety signal of the most basic type. When the cell has fully adequate supplies of ATP, a high mitochondrial membrane voltage and a deeply reduced CoQ couple then ROS generation becomes almost inevitable.
It is a core, deep level, simple system. How it works is the subject of the Protons thread of posts, as is how it malfunctions.
I find it very, very difficult to envisage that the control of ingress of calories on a whole body basis (hunger/satiety) is not similarly integrated around an ROS generating system within dedicated neurons of the brain, with the hypothalamus being the most likely location.
I've had this as a persistent suspicion for a very long time but you can't get around to reading about everything at the same time. And I have to admit that neurological thinking about hunger and satiety has always struck me as a highly disreputable field. Insulin and satiety smell like cholesterol and the lipid hypothesis.
So yesterday I finally went and had a look for a reasonably recent review of ROS within the hypothalamus and this one came up pretty high in the PubMed listing
Impact of hypothalamic reactive oxygen species in the regulation of energy metabolism and food intake
This gives a flavour:
"Thus, it appears that NPY/AgRP neurons activation is mediated by a decrease in ROS levels while POMC neurons activation is driven by ROS (Andrews et al., 2008). Indeed, icv administration of ROS scavengers induces significantly lower c-Fos expression in POMC neurons and increases food intake during light cycle, observed via an increase of c-Fos expression in AgRP/NPY neurons (Diano et al., 2011). Similarly, addition of H2O2 depolarizes POMC neurons, increases the firing rate, and an icv injection of H2O2 causes significantly less feeding of mice after an overnight fast".
A lot of the work cited is not terribly well performed and no one has the Protons framework to slot their findings in to, but it's a start. What I am looking for is that these cell types do express GLUT proteins and CD36 proteins. I would expect them to be sampling arterial blood or CSF and integrating NADH and FADH2 inputs to their mitochondrial electron transport chain to "decide" whether there are adequate calories to consider that satiety or hunger might be the preferred descriptor of the body's current state.
Whether these cells express insulin receptors to facilitate ingress of substrate is something to be picked at. As I am completely biased against the concept that insulin is a satiety hormone, I would prefer this not to be the case but may be wrong. Time will tell. It looks logical to me that the brain would look at the nutrient levels present in excess of those being disposed of by peripheral insulin in to peripheral cells. As large numbers of peripheral cells become "full" under the influence of insulin, the brain should pick up the rising level of excess nutrients as the signal to call a halt on hunger. Doing this within the brain shouldn't need insulin, merely a set of relatively low affinity transporters to allow glucose and lipid uptake as insulin completes its peripheral function. Satiety should be picked up when enough cells have enough calories, whole body, that they no longer behave as a calorie "sump". The job of the brain is to pick up evidence from the nutrient levels that the sump is full and satiety can be declared.
For hunger, high affinity transporters would allow ROS to be generated easily and falling ROS would signal that energy availability was low.
These signals will come from the neural mitochondrial ETC generating ROS. The best ROS generating nutrients will be the most satiating. Saturated fats spring to mind if you follow the Protons thread.
Obviously there are whole load more hormones which can influence the generation of ROS within neurons. Physiology has applied layers and layers of signalling to maximise reproductive fitness. I have minimal interest in these "higher level" signals. They are there, they will modulate the core process I've been talking about but I see no way that they will do anything fundamentally different from or in opposition to the ROS system.
I'll give the rest of the review a bit of a read and see if it's worth posting about.
Peter
Friday, November 08, 2019
Insulin makes you hungry (11) But not in Denmark?
Preamble. The best papers are those which challenge your ideas. When they conflict with what appears to be very hard evidence which support your mindset they become really exciting. Sometimes you just have to shrug, label the new finding as important and put it on the back burner to be ruminated about over the coming months. Sometimes a potential explanation is possible. This post is essentially a fairytale set in Denmark. It may be completely wrong. Or not. Here we go.
This paper came up in the comments to the last post from Gabor Erdosi via raphi. It is from Astrup's group in Denmark and I have to say I have a lot of time for Prof Astrup as he was one of the more influential people who objected to the gross stupidity of Denmark's transient fat tax a few years ago. The fat tax was abandoned quite rapidly as sensible EU dwelling Danes merely popped across their open border and stocked up with un-fat-taxed butter in Germany. Anyway:
The role of postprandial releases of insulin and incretin hormones in meal-induced satiety-effect of obesity and weight reduction
This is the crucial graph
Take 12 lean people, feed them a 600kcal sandwich for breakfast, wait just over three hours then offer them an ad-lib, well mixed pasta salad and see how much of this they eat.
The higher their insulin went after breakfast, the less they ate at lunchtime.
Insulin exposure is clearly associated with reduced subsequent food intake. You might be tempted to assume causality here, but you can't. It's an observational study of a very specific set of people. It can be used to generate an hypothesis, such as insulin suppresses subsequent food intake. But then you would have to test that hypothesis.
You could also simply go back through the literature to interventional studies which actually imposed changes in insulin levels and come to the opposite conclusion. Rodin et al did this here
Effect of insulin and glucose on feeding behavior
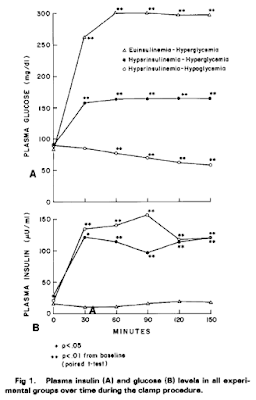
which makes that particular hypothesis untenable. Insulin makes you hungry.
Here are the core findings, clamp values first
Here are the hunger ratings
and the amount of liquid food consumed, via a straw, through a screen:
To me personally, his study is very convincing. The principle is simple, logical and comprehensible. I would have been happier if he had also tracked FFAs in the study but we all know what insulin does to FFA levels (in the absence of fat ingestion). My personal view is that the brain looks at the availability of calories. Normoglycaemia with rapidly falling FFAs (the effect of insulin on adipocytes) is going to generate hunger. No one would expect any different. The action of insulin is the inhibition of lipolysis. Much higher levels are needed to facilitate the uptake of glucose.
We have a paradox, excellent. Direct insulin infusion makes you hungry. Insulin response to food makes you less hungry.
That is so cool.
Sooooo. Is it even remotely possible to explain the observation picked up by Prof Astrup in his 12 lean Danes? Starting from the basis that insulin drives calorie loss in to adipocytes with subsequent hunger? Speculation warning.
This group of Danes is very unusual. They have lived, on average, for 34 years in modern Denmark and they are not over weight. They have never counted a calorie, never been to Weight W@tchers, never had an eating disorder. They eat as much as they are comfortable with and eat again when they are hungry. If they pig out at Christmas they don't need to go on a diet in the New Year. They put zero effort in to being lean. That is a very special sort of person. They have normal appetite control.
When we give them a fixed calorie breakfast the insulin response varies. With these normal people I think it is a reasonable assumption that if the 600kcal is high compared to their preferred size of breakfast, the insulin level will go higher. There is more food than needed so more to store, that needs more insulin.
Members of the group who fancied many more than 600kcal for breakfast will have produced a low insulin response to the 600kcal specified by the study.
Now, it gets interesting because you cannot remotely account for a 1200kcal (3000kJ) difference in lunchtime eating by speculating about preferred breakfast size. The effect is too big.
The storage of calories is the simplest of actions of insulin. It does other things too. For these we have to go to the very, very artificial model of Veech's isolated working rat hearts. However some of the findings do have some bearing on real life.
In this paper
Substrate signaling by insulin: a ketone bodies ratio mimics insulin action in heart
we have this snippet:
"Unexpectedly, insulin increased cardiac hydraulic work but decreased net glycolytic flux and O2 consumption, improving net cardiac efficiency by 28%".
Insulin facilitates glucose diffusion in to the myocytes but partitions it in to stored glycogen. Glycolysis actually decreases but there is an increase in efficiency which gives the 28% increase in cardiac work.
Let that sink in. Insulin makes energy production more efficient while diverting calories in to storage. If you wanted to fatten someone up that seems like a good plan.
From a related paper by Veech's group
Insulin, ketone bodies, and mitochondrial energy transduction
we have a slight elaboration:
"The increase in efficiency associated with insulin administration is not readily explained by such a straightforward mechanism [as for ketones]; other factors, such as reduction of the mitochondrial NAD couple or specific effects like covalent modification of mitochondrial membrane protein, will have to be considered as possible factors altering efficiency of ATP synthesis".
Veech's model runs on glucose alone but there will undoubtedly be residual FFAs in the cardiac myocytes. You just have to wonder whether the effect of insulin is to extract these from the mitochondrial uncoupling proteins and covalently bind them in to intracellular triglycerides. An interesting idea and it would certainly tighten the coupling of the ETC.
Bottom line: Insulin, when working as it should, diverts calories to storage but increases efficiency of energy production to allow normal metabolism. I hold that this happens in the elevated insulin individuals of the lean subject group. Recall that all of these people are naturally lean. When the insulin wears off they realise, metabolically, that they have gained a (very) little weight while running a very efficient metabolism. If they have extra stored calories which, being naturally lean, they don't need, why should they eat much at lunch time?
The hypoinsulinaemic lean people get their 600kcal, decide it is way too little to bother storing and partition it towards utilisation. There is no drive towards storage, very little insulin, no insulin mediated increased efficiency. Substrate is available, it gets oxidised. Very little gets stored. This is the low insulin state. When lunch is presented to this naturally weight stable person their metabolism realises that it has not maintained fat stores after the 600kcal breakfast so they eat more at lunch time.
We have a period of high efficiency calorie conservation in the high insulin group and a period of low efficiency calorie wastage in the low insulin individuals. Because these people have that rare gem, a functional metabolism, they simply adjust subsequent food intake to reflect their previous fuel partitioning during the three hours from breakfast to lunch.
It is perfectly reasonable to mistake this scenario as an indicator that insulin is a satiety signal. Easily done. It's incorrect.
Peter
The invariable after-thought: Does insulin correlate inversely with reduced food intake in either the obese group at the start of the study or after marked weight loss?
No. Of course not.
This paper came up in the comments to the last post from Gabor Erdosi via raphi. It is from Astrup's group in Denmark and I have to say I have a lot of time for Prof Astrup as he was one of the more influential people who objected to the gross stupidity of Denmark's transient fat tax a few years ago. The fat tax was abandoned quite rapidly as sensible EU dwelling Danes merely popped across their open border and stocked up with un-fat-taxed butter in Germany. Anyway:
The role of postprandial releases of insulin and incretin hormones in meal-induced satiety-effect of obesity and weight reduction
This is the crucial graph
Take 12 lean people, feed them a 600kcal sandwich for breakfast, wait just over three hours then offer them an ad-lib, well mixed pasta salad and see how much of this they eat.
The higher their insulin went after breakfast, the less they ate at lunchtime.
Insulin exposure is clearly associated with reduced subsequent food intake. You might be tempted to assume causality here, but you can't. It's an observational study of a very specific set of people. It can be used to generate an hypothesis, such as insulin suppresses subsequent food intake. But then you would have to test that hypothesis.
You could also simply go back through the literature to interventional studies which actually imposed changes in insulin levels and come to the opposite conclusion. Rodin et al did this here
Effect of insulin and glucose on feeding behavior
which makes that particular hypothesis untenable. Insulin makes you hungry.
Here are the core findings, clamp values first
Here are the hunger ratings
and the amount of liquid food consumed, via a straw, through a screen:
To me personally, his study is very convincing. The principle is simple, logical and comprehensible. I would have been happier if he had also tracked FFAs in the study but we all know what insulin does to FFA levels (in the absence of fat ingestion). My personal view is that the brain looks at the availability of calories. Normoglycaemia with rapidly falling FFAs (the effect of insulin on adipocytes) is going to generate hunger. No one would expect any different. The action of insulin is the inhibition of lipolysis. Much higher levels are needed to facilitate the uptake of glucose.
We have a paradox, excellent. Direct insulin infusion makes you hungry. Insulin response to food makes you less hungry.
That is so cool.
Sooooo. Is it even remotely possible to explain the observation picked up by Prof Astrup in his 12 lean Danes? Starting from the basis that insulin drives calorie loss in to adipocytes with subsequent hunger? Speculation warning.
This group of Danes is very unusual. They have lived, on average, for 34 years in modern Denmark and they are not over weight. They have never counted a calorie, never been to Weight W@tchers, never had an eating disorder. They eat as much as they are comfortable with and eat again when they are hungry. If they pig out at Christmas they don't need to go on a diet in the New Year. They put zero effort in to being lean. That is a very special sort of person. They have normal appetite control.
When we give them a fixed calorie breakfast the insulin response varies. With these normal people I think it is a reasonable assumption that if the 600kcal is high compared to their preferred size of breakfast, the insulin level will go higher. There is more food than needed so more to store, that needs more insulin.
Members of the group who fancied many more than 600kcal for breakfast will have produced a low insulin response to the 600kcal specified by the study.
Now, it gets interesting because you cannot remotely account for a 1200kcal (3000kJ) difference in lunchtime eating by speculating about preferred breakfast size. The effect is too big.
The storage of calories is the simplest of actions of insulin. It does other things too. For these we have to go to the very, very artificial model of Veech's isolated working rat hearts. However some of the findings do have some bearing on real life.
In this paper
Substrate signaling by insulin: a ketone bodies ratio mimics insulin action in heart
we have this snippet:
"Unexpectedly, insulin increased cardiac hydraulic work but decreased net glycolytic flux and O2 consumption, improving net cardiac efficiency by 28%".
Insulin facilitates glucose diffusion in to the myocytes but partitions it in to stored glycogen. Glycolysis actually decreases but there is an increase in efficiency which gives the 28% increase in cardiac work.
Let that sink in. Insulin makes energy production more efficient while diverting calories in to storage. If you wanted to fatten someone up that seems like a good plan.
From a related paper by Veech's group
Insulin, ketone bodies, and mitochondrial energy transduction
we have a slight elaboration:
"The increase in efficiency associated with insulin administration is not readily explained by such a straightforward mechanism [as for ketones]; other factors, such as reduction of the mitochondrial NAD couple or specific effects like covalent modification of mitochondrial membrane protein, will have to be considered as possible factors altering efficiency of ATP synthesis".
Veech's model runs on glucose alone but there will undoubtedly be residual FFAs in the cardiac myocytes. You just have to wonder whether the effect of insulin is to extract these from the mitochondrial uncoupling proteins and covalently bind them in to intracellular triglycerides. An interesting idea and it would certainly tighten the coupling of the ETC.
Bottom line: Insulin, when working as it should, diverts calories to storage but increases efficiency of energy production to allow normal metabolism. I hold that this happens in the elevated insulin individuals of the lean subject group. Recall that all of these people are naturally lean. When the insulin wears off they realise, metabolically, that they have gained a (very) little weight while running a very efficient metabolism. If they have extra stored calories which, being naturally lean, they don't need, why should they eat much at lunch time?
The hypoinsulinaemic lean people get their 600kcal, decide it is way too little to bother storing and partition it towards utilisation. There is no drive towards storage, very little insulin, no insulin mediated increased efficiency. Substrate is available, it gets oxidised. Very little gets stored. This is the low insulin state. When lunch is presented to this naturally weight stable person their metabolism realises that it has not maintained fat stores after the 600kcal breakfast so they eat more at lunch time.
We have a period of high efficiency calorie conservation in the high insulin group and a period of low efficiency calorie wastage in the low insulin individuals. Because these people have that rare gem, a functional metabolism, they simply adjust subsequent food intake to reflect their previous fuel partitioning during the three hours from breakfast to lunch.
It is perfectly reasonable to mistake this scenario as an indicator that insulin is a satiety signal. Easily done. It's incorrect.
Peter
The invariable after-thought: Does insulin correlate inversely with reduced food intake in either the obese group at the start of the study or after marked weight loss?
No. Of course not.
Sunday, November 03, 2019
Insulin makes you hungry (10) Processing
I picked up a link to this paper from an image of a table, somewhere on Tinternet. I didn't realise which paper it was. It was really interesting from the metabolic point of view and I kept looking through the results section to find the post-prandial metabolic effect of ultra-processed foods vs unprocessed foods. You know, the effect which might determine where the calories from a given food might end up, either available for metabolism versus lost in to adipocytes.
Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake
This is from the introduction
"Ultra-processed foods may facilitate overeating and the development of obesity (Poti et al., 2017) because they are typically high in calories, salt, sugar, and fat (Poti et al., 2015) and have been suggested to be engineered to have supernormal appetitive properties (Kessler, 2009; Moss, 2013; Moubarac, 2015; Schatzker, 2015). Furthermore, ultra-processed foods are theorized to disrupt gut-brain signaling and may influence food reinforcement and overall intake via mechanisms distinct from the palatability or energy density of the food (Small and DiFeliceantonio, 2019)"
What this is saying is that making foods high in calories + "supernormal appetitive properties" makes you eat more, so you become fat. And that they alter gut brain signalling to increase "reinforcement" and so increase intake. You have to put the excess calories somewhere so you get fat.
Supernormal appetitive properties. Energy density. Reinforcement.
Psychobabble.
But the bottom line is that highly processed foods do produce steady weight gain over two weeks and low processed foods do the opposite.
What we need to know is how a given food affects both the acute and chronic metabolic response, especially insulin levels and signalling. That's not going to come from the psychobabblers.
Luckily there are groups with an interest in the metabolic effects of food, rather than being lost in un-Rewarding theories of reinforcement. Like this group:
Does food insulin index in the context of mixed meals affect postprandial metabolic responses and appetite in obese adolescents with insulin resistance? A randomised cross-over trial
They compared two meals with the same low glycaemic index (GI) but differing insulin secretion indices (Insulin index, II). Both meals had very similar macros and they look suspiciously like processed vs unprocessed. Insulin (and glucose) were higher after the high II meals:
The low II meals generated less hunger in the post prandial period. I'm not sure what hunger has to do with weight gain, I get the impression from people like Hall et al that calorie intake controls weight gain. Hunger is something else, will-power dependent maybe.
The second study has some problems too. The low II food did not taste as good as the high II food. So Rewarders have a lifeline here. And if you are making a marketable commodity to be labelled as "food", anything you can do to increase its palatability is going to improve sales. If palatability should turn out to be intrinsically linked to a food's II then you are set to drive hunger combined with those increased sales. Win-win for the shareholders but not so much for the consumers. No one wants to be fat. Equally, no one can tolerate being hungry...
The second problem is that there was no increase in food intake for the high II group during the end-of-study buffet. An increase in food intake here would have been nice, to corroborate the increased hunger. But that meal was a free choice buffet being offered to people who were already obese, so poor food choices may well have over-ridden the differences in experimental meal induced hunger. Back in the days of better designed studies the post-insulin test meal was a uniform soup-like liquid, consumed via a straw through a screen so there were no visual or food choice cues to influence food intake. A pity, but there you go.
Insulin makes you hungry. Not directly, but by diverting calories in to storage. You lose those calories so your brain gets hungry. Making you fat is how insulin makes you hungry. Pure CICO but the calories-out go in to your adipocytes...
Peter
Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake
This is from the introduction
"Ultra-processed foods may facilitate overeating and the development of obesity (Poti et al., 2017) because they are typically high in calories, salt, sugar, and fat (Poti et al., 2015) and have been suggested to be engineered to have supernormal appetitive properties (Kessler, 2009; Moss, 2013; Moubarac, 2015; Schatzker, 2015). Furthermore, ultra-processed foods are theorized to disrupt gut-brain signaling and may influence food reinforcement and overall intake via mechanisms distinct from the palatability or energy density of the food (Small and DiFeliceantonio, 2019)"
What this is saying is that making foods high in calories + "supernormal appetitive properties" makes you eat more, so you become fat. And that they alter gut brain signalling to increase "reinforcement" and so increase intake. You have to put the excess calories somewhere so you get fat.
Supernormal appetitive properties. Energy density. Reinforcement.
Psychobabble.
But the bottom line is that highly processed foods do produce steady weight gain over two weeks and low processed foods do the opposite.
What we need to know is how a given food affects both the acute and chronic metabolic response, especially insulin levels and signalling. That's not going to come from the psychobabblers.
Luckily there are groups with an interest in the metabolic effects of food, rather than being lost in un-Rewarding theories of reinforcement. Like this group:
Does food insulin index in the context of mixed meals affect postprandial metabolic responses and appetite in obese adolescents with insulin resistance? A randomised cross-over trial
They compared two meals with the same low glycaemic index (GI) but differing insulin secretion indices (Insulin index, II). Both meals had very similar macros and they look suspiciously like processed vs unprocessed. Insulin (and glucose) were higher after the high II meals:
The low II meals generated less hunger in the post prandial period. I'm not sure what hunger has to do with weight gain, I get the impression from people like Hall et al that calorie intake controls weight gain. Hunger is something else, will-power dependent maybe.
The second study has some problems too. The low II food did not taste as good as the high II food. So Rewarders have a lifeline here. And if you are making a marketable commodity to be labelled as "food", anything you can do to increase its palatability is going to improve sales. If palatability should turn out to be intrinsically linked to a food's II then you are set to drive hunger combined with those increased sales. Win-win for the shareholders but not so much for the consumers. No one wants to be fat. Equally, no one can tolerate being hungry...
The second problem is that there was no increase in food intake for the high II group during the end-of-study buffet. An increase in food intake here would have been nice, to corroborate the increased hunger. But that meal was a free choice buffet being offered to people who were already obese, so poor food choices may well have over-ridden the differences in experimental meal induced hunger. Back in the days of better designed studies the post-insulin test meal was a uniform soup-like liquid, consumed via a straw through a screen so there were no visual or food choice cues to influence food intake. A pity, but there you go.
Insulin makes you hungry. Not directly, but by diverting calories in to storage. You lose those calories so your brain gets hungry. Making you fat is how insulin makes you hungry. Pure CICO but the calories-out go in to your adipocytes...
Peter
Subscribe to:
Posts (Atom)