Tucker and Mike both gave me the heads-up on this paper recently.
Linoleic acid causes greater weight gain than saturated fat without hypothalamic inflammation in the male mouse
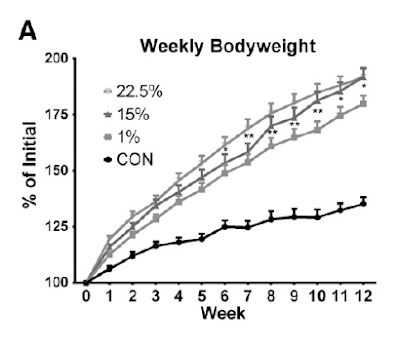
It's nice and simple: feeding 22.5% of calories as linoleic acid (LA) to a mouse makes it obese and insulin resistant. Feeding 15% of calories as LA makes it identically obese but without the insulin resistance. The argument can be made, very convincingly, that LA is converts to 4-hydroxynonenyl (4-HNE) in proportion to the LA content of the diet. 4-HNE is a powerful driver of insulin resistance so the hypoglycaemic response to exogenous insulin is markedly blunted in the 22.5% LA group of mice. As in the top line here:
Here are the weight gains, which need a little consideration:
With the eye of faith you can see that the top line (22.5% LA) starts off gaining weight faster than the next line down (15% LA) but converges from around 7-8 weeks onward. My guess is that this is when insulin resistance from 4-HNE started to over-ride the insulin sensitising effect of LA which caused the weight gain in the first place.
Now the third line down is the interesting one. This diet only contained 1% linoleic acid. OK, these mice are statistically significantly slimmer than the 15% and 22.5% LA fed mice (p less than 0.05) but they are hardly exactly svelte when compared to the crapinabag (CIAB)-fed control mice (black line down at the bottom). And the CIAB food contained 4.22% of calories derived from LA.
That needs some thinking about.
It makes me ask: Why do the vast majority of high fat fed mice/rats become obese? Apart from the fact that they have been selected for this response.
There's probably another post or two needed on that one.
Peter
As an aside I would just comment that while I agree with Tucker that 4-HNE and related products of free radical modified PUFA are the best explanation for this study, my feeling is that the 15% LA group with sustained insulin sensitivity allowing sustained weight gain probably explains the situation in the current human population rather better. As sustained adipocyte distension progresses then we eventually get FFAs released in the presence of glucose and insulin, a Bad Thing. The ROS from this combination will eventually generate 4-HNE too but rather further down the obesity road compared with the 22.5% LA situation.
Monday, December 31, 2018
Thursday, December 20, 2018
Urinary c-peptide
It's the Winter Solstice tomorrow, greetings to all! My favourite astronomical event of the year, even though we do the major feasting on Christmas day in our house. Anyway, here's a fairytale for the depths of Winter (in the northern hemisphere anyway). Happy Solstice!
*********
Here we go: Just occasionally you come across a statement like this:
"(B) Insulin secretion throughout the day was assessed by 24-hr urinary c-peptide excretion and was significantly reduced only following the RC diet".
Okay, what does it suggest to us when the reduced 24-hr urinary c-peptide group lost less fat than the higher c-peptide group? Less insulin but less fat loss??? Perhaps it suggests that the insulin hypothesis of obesity is incorrect?
C-peptide is part of pro-insulin. Each molecule of insulin produced provides one molecule of c-peptide within the pancreas. Assuming (not completely accurately) that c-peptide is not consumed within the body we can use its 24-hr average urinary excretion as a surrogate for overall insulin production. With an awful lot of caveats, this seems fair to me. I think statement B is correct.
So 24-hr urinary c-peptide gives us an idea of how many molecules of insulin are being manufactured per day by the islets within the pancreas. Insulin is broken down by insulin degrading enzyme as part of its signalling process, not exactly proportionally, but as a general principle this is correct. I went through it in some detail when thinking about the Potato Diet, a sub-category of carbosis. The more signalling, the more degradation.
On average around 50% of secreted insulin (in dogs on a mixed diet) is removed by the liver on first passage from the portal vein through to the hepatic vein (termed first past extraction, FPE). Humans are very similar. None of this hepatic first pass extracted insulin ever arrives in the general circulation. The rate of extraction varied from as low as just over 20% up to almost 80% in the dog study. If you have 100 molecules of c-peptide produced, somewhere between 20 and 80 of the associated molecules of insulin will never arrive in the systemic circulation.
Does anything specific alter the FPE? Well, yes, of course. Does anyone think it might be free fatty acid delivery to the liver? Much as this paper tells us
Free Fatty Acids Impair Hepatic Insulin Extraction in Vivo
So under a weight stable modest LC diet (or more accurately; under whole body adipose tissue mass stability) the reduction of insulin secretion from the pancreas under that modestly reduced carbohydrate intake will undoubtedly occur, but would be offset by reduced hepatic FPE and enough insulin will penetrate to adipocytes to keep them full. In the real world this is extremely difficult to make happen, people just want to eat less on a LC diet because as insulin falls more fat exits adipocytes and hunger diminishes. As in Aberdeen. But you can approximate it by artificially controlling (increasing) food intake, ie you pay people to eat more dietary fat than they would like to (which keeps insulin secretion unchanged but increases fatty acid delivery to the liver), hepatic FPE falls and more insulin reaches adipocytes to keep them full.
Under ketosis (let's say with carbs less than 20g/d) there is so little insulin secretion that having an FPE which is probably approaching zero doesn't matter much. Near basal physiological insulin is secreted, almost none is FPE-ed by the liver but there is still minimal exposure of adipocytes to insulin because almost none is being secreted in the first place. So appetite plummets as FFAs rise as they pour out from the adipocytes, despite a minimal hepatic FPE. This should make it even harder to overeat. However, if you do manage it, minimal hepatic FPE by the liver is one of your methods to maintain fat stores under ketosis!
Conversely you can achieve low FFA delivery to the liver by using acipimox. People do not necessarily gain weight with this lipolysis inhibiting drug because it decreases hepatic FFA delivery, so increases hepatic insulin signalling and so increases hepatic insulin metabolism. I assume this increased insulin metabolism will increase FPE and this will decrease insulin delivery to the systemic circulation. I also think this is also how carbosis works, hence the need for very low fat provision in any carbosis inducing diet.
Anyway, here's a thought experiment using made-up numbers. Any resemblance to real life is totally accidental. If a moderate carb diet (say 140g/d) allows a 22% fall in 24-hr urinary c-peptide, does this mean there is a 22% fall in 24h exposure of adipocytes to systemic insulin? Well no...
Say 100 molecules of insulin are secreted and FPE pre-study is 50% then 50 molecules of insulin survive passage through the liver to suppress systemic lipolysis. If only 78 molecules of insulin are secreted under mild carbohydrate restriction but FPE falls to 20% (exaggeration to make the point!) due to increased lipolysis delivering extra FFAs to the liver, then 62 molecules of insulin will make it through FPE and as far as the adipocytes. This insulin could conceivably allow less lipolysis when compared to a carbosis inducing diet, despite reducing insulin secretion.
On a diet in which fat is so restricted as to allow almost none to be spared for oxidation so that FFA delivery to the liver falls precipitously, we can suggest an 80% FPE might be the result. This would be the situation under carbosis, say with a human eating 7.7% fat as part of a severely calorie restricted diet. So of the 100 molecules of insulin still being produced under these circumstances (typified by no fall in 24-hr urinary c-peptide) with an 80% FPE only 20 of those molecules of insulin will eventually hit the adipocytes and so lipolysis would then be greater than under the modest LC diet.
Please bear in mind that these numbers are a reductio ad absurdum example, but they do make a point about what is possible. There are other effects which would kick in but that's not my point here.
My grossly biased opinion is that any study which shows an intervention with superiority in fat loss will be associated with either lower insulin exposure of adipocytes or with an induced failure of insulin to act on those adipocytes (ie by metformin, alcohol, fructose or of course palmitic acid, plus a few others) than is achieved by any comparison diet.
But then I would say that..........
Peter
*********
Here we go: Just occasionally you come across a statement like this:
"(B) Insulin secretion throughout the day was assessed by 24-hr urinary c-peptide excretion and was significantly reduced only following the RC diet".
Okay, what does it suggest to us when the reduced 24-hr urinary c-peptide group lost less fat than the higher c-peptide group? Less insulin but less fat loss??? Perhaps it suggests that the insulin hypothesis of obesity is incorrect?
C-peptide is part of pro-insulin. Each molecule of insulin produced provides one molecule of c-peptide within the pancreas. Assuming (not completely accurately) that c-peptide is not consumed within the body we can use its 24-hr average urinary excretion as a surrogate for overall insulin production. With an awful lot of caveats, this seems fair to me. I think statement B is correct.
So 24-hr urinary c-peptide gives us an idea of how many molecules of insulin are being manufactured per day by the islets within the pancreas. Insulin is broken down by insulin degrading enzyme as part of its signalling process, not exactly proportionally, but as a general principle this is correct. I went through it in some detail when thinking about the Potato Diet, a sub-category of carbosis. The more signalling, the more degradation.
On average around 50% of secreted insulin (in dogs on a mixed diet) is removed by the liver on first passage from the portal vein through to the hepatic vein (termed first past extraction, FPE). Humans are very similar. None of this hepatic first pass extracted insulin ever arrives in the general circulation. The rate of extraction varied from as low as just over 20% up to almost 80% in the dog study. If you have 100 molecules of c-peptide produced, somewhere between 20 and 80 of the associated molecules of insulin will never arrive in the systemic circulation.
Does anything specific alter the FPE? Well, yes, of course. Does anyone think it might be free fatty acid delivery to the liver? Much as this paper tells us
Free Fatty Acids Impair Hepatic Insulin Extraction in Vivo
So under a weight stable modest LC diet (or more accurately; under whole body adipose tissue mass stability) the reduction of insulin secretion from the pancreas under that modestly reduced carbohydrate intake will undoubtedly occur, but would be offset by reduced hepatic FPE and enough insulin will penetrate to adipocytes to keep them full. In the real world this is extremely difficult to make happen, people just want to eat less on a LC diet because as insulin falls more fat exits adipocytes and hunger diminishes. As in Aberdeen. But you can approximate it by artificially controlling (increasing) food intake, ie you pay people to eat more dietary fat than they would like to (which keeps insulin secretion unchanged but increases fatty acid delivery to the liver), hepatic FPE falls and more insulin reaches adipocytes to keep them full.
Under ketosis (let's say with carbs less than 20g/d) there is so little insulin secretion that having an FPE which is probably approaching zero doesn't matter much. Near basal physiological insulin is secreted, almost none is FPE-ed by the liver but there is still minimal exposure of adipocytes to insulin because almost none is being secreted in the first place. So appetite plummets as FFAs rise as they pour out from the adipocytes, despite a minimal hepatic FPE. This should make it even harder to overeat. However, if you do manage it, minimal hepatic FPE by the liver is one of your methods to maintain fat stores under ketosis!
Conversely you can achieve low FFA delivery to the liver by using acipimox. People do not necessarily gain weight with this lipolysis inhibiting drug because it decreases hepatic FFA delivery, so increases hepatic insulin signalling and so increases hepatic insulin metabolism. I assume this increased insulin metabolism will increase FPE and this will decrease insulin delivery to the systemic circulation. I also think this is also how carbosis works, hence the need for very low fat provision in any carbosis inducing diet.
Anyway, here's a thought experiment using made-up numbers. Any resemblance to real life is totally accidental. If a moderate carb diet (say 140g/d) allows a 22% fall in 24-hr urinary c-peptide, does this mean there is a 22% fall in 24h exposure of adipocytes to systemic insulin? Well no...
Say 100 molecules of insulin are secreted and FPE pre-study is 50% then 50 molecules of insulin survive passage through the liver to suppress systemic lipolysis. If only 78 molecules of insulin are secreted under mild carbohydrate restriction but FPE falls to 20% (exaggeration to make the point!) due to increased lipolysis delivering extra FFAs to the liver, then 62 molecules of insulin will make it through FPE and as far as the adipocytes. This insulin could conceivably allow less lipolysis when compared to a carbosis inducing diet, despite reducing insulin secretion.
On a diet in which fat is so restricted as to allow almost none to be spared for oxidation so that FFA delivery to the liver falls precipitously, we can suggest an 80% FPE might be the result. This would be the situation under carbosis, say with a human eating 7.7% fat as part of a severely calorie restricted diet. So of the 100 molecules of insulin still being produced under these circumstances (typified by no fall in 24-hr urinary c-peptide) with an 80% FPE only 20 of those molecules of insulin will eventually hit the adipocytes and so lipolysis would then be greater than under the modest LC diet.
Please bear in mind that these numbers are a reductio ad absurdum example, but they do make a point about what is possible. There are other effects which would kick in but that's not my point here.
My grossly biased opinion is that any study which shows an intervention with superiority in fat loss will be associated with either lower insulin exposure of adipocytes or with an induced failure of insulin to act on those adipocytes (ie by metformin, alcohol, fructose or of course palmitic acid, plus a few others) than is achieved by any comparison diet.
But then I would say that..........
Peter
Wednesday, December 12, 2018
A post not about Walter Kempner
I've had this paper on my hard drive for a while. It's been sitting somewhere near the front of the back of my mind but was doing nothing to really grab my interest.
Chronic high-sucrose diet increases fibroblast growth factor 21 production and energy expenditure in mice
I've got a draft of a post from mid summer this year which I wrote simply because I like the attitude of the authors. They say things like:
"Excess carbohydrate intake causes obesity in humans".
That's the first line of the abstract. You know, it's that "nailing your colours to the mast" sort of a statement. Even though I do think life is a little more complex than that.
Anyway, I like these folks who are looking at the slimming effect of sucrose in BL6 mice. That's correct, sucrose is a slimming drug/food in mice, under the correct circumstances. People too. The data in the 2017 paper is an extension of the work they did in 2012, written up in this paper:
Ingestion of a moderate high‐sucrose diet results in glucose intolerance with reduced liver glucokinase activity and impaired glucagon‐like peptide‐1 secretion
I don't intend to go through either paper in detail, it's just that the 2012 paper has some rather special macro ratios that caught my eye.
This is what they did to the mice in that original paper:
"After adaptation for 2 weeks, they [the mice] were divided into three groups and fed a normal chow diet (NC), a high‐starch diet (ST) supplemented with 38.5% corn starch or a SUC containing 38.5% sucrose; the latter two diets were prepared by the addition of corn starch or sucrose, respectively, to CE‐2 (Table 1)"
Essentially they are diluting chow with starch or sucrose. Here is Table 1 for the diet compositions, note my red rectangle:
With group sizes of n=4 and five weeks on the diet very little of anything reached statistical or biological significance. The 2017 study used a slightly modified version of the diet to keep a low fat percentage identical across the diets but still had 38.5% of calories from sucrose, was run for 15 weeks and had group sizes of n=8-10. Results were statistically significant all over the place and suggest that the sucrose diet is decidedly good for metabolic health and gives a slim phenotype on ad lib consumption. Just so long as fat calories are very, very low. This looks very much like what Denise Minger described as carbosis, based in part around Walter Kempner's very effective, very unpleasant, ultra low fat, high sucrose medical diet. The Rice Diet is very real.
This post is not about any of the above.
Now, watch carefully. I'm going to sneak in some more macros
If you wanted a "reduced" fat diet which induces carbosis in human beings I recon the red text is pretty well it. I particularly enjoyed that exactly 7.7% of calories came from fat in each diet, this could almost be deliberate. If you combine what is almost certainly a very effective spontaneous weight loss diet with a 30% calorie restriction I suspect you might be on to a winner when comparing it against a reduced carbohydrate diet. Of course to really nail it you would have to compare it to an absolutely non ketogenic diet, say one supplying a total of 140g/d of carbohydrate. Does carbosis beat a middling carbohydrate mixed diet? You bet.
Oh, the scribbled-in red numbers came from Table 2 of this paper.
Most people in respectable CICO based mainstream nutrition have never heard of carbosis, Walter Kempner, the Rice Diet and have probably never heard of Denise Minger.
But Kevin Hall has. My respect for his knowledge-base and ingenuity is vast. Such a pity it's wasted on constructing props for his bizarre pet theories of weight control.
While the 7.7% of calories as fat in both studies is something which amuses me greatly, I do have to admit it may just be an hysterical accident.
At least I'm up front about my rather pronounced personal biases and rather peculiar sense of humour.
Peter
Chronic high-sucrose diet increases fibroblast growth factor 21 production and energy expenditure in mice
I've got a draft of a post from mid summer this year which I wrote simply because I like the attitude of the authors. They say things like:
"Excess carbohydrate intake causes obesity in humans".
That's the first line of the abstract. You know, it's that "nailing your colours to the mast" sort of a statement. Even though I do think life is a little more complex than that.
Anyway, I like these folks who are looking at the slimming effect of sucrose in BL6 mice. That's correct, sucrose is a slimming drug/food in mice, under the correct circumstances. People too. The data in the 2017 paper is an extension of the work they did in 2012, written up in this paper:
Ingestion of a moderate high‐sucrose diet results in glucose intolerance with reduced liver glucokinase activity and impaired glucagon‐like peptide‐1 secretion
I don't intend to go through either paper in detail, it's just that the 2012 paper has some rather special macro ratios that caught my eye.
This is what they did to the mice in that original paper:
"After adaptation for 2 weeks, they [the mice] were divided into three groups and fed a normal chow diet (NC), a high‐starch diet (ST) supplemented with 38.5% corn starch or a SUC containing 38.5% sucrose; the latter two diets were prepared by the addition of corn starch or sucrose, respectively, to CE‐2 (Table 1)"
Essentially they are diluting chow with starch or sucrose. Here is Table 1 for the diet compositions, note my red rectangle:
With group sizes of n=4 and five weeks on the diet very little of anything reached statistical or biological significance. The 2017 study used a slightly modified version of the diet to keep a low fat percentage identical across the diets but still had 38.5% of calories from sucrose, was run for 15 weeks and had group sizes of n=8-10. Results were statistically significant all over the place and suggest that the sucrose diet is decidedly good for metabolic health and gives a slim phenotype on ad lib consumption. Just so long as fat calories are very, very low. This looks very much like what Denise Minger described as carbosis, based in part around Walter Kempner's very effective, very unpleasant, ultra low fat, high sucrose medical diet. The Rice Diet is very real.
This post is not about any of the above.
Now, watch carefully. I'm going to sneak in some more macros
If you wanted a "reduced" fat diet which induces carbosis in human beings I recon the red text is pretty well it. I particularly enjoyed that exactly 7.7% of calories came from fat in each diet, this could almost be deliberate. If you combine what is almost certainly a very effective spontaneous weight loss diet with a 30% calorie restriction I suspect you might be on to a winner when comparing it against a reduced carbohydrate diet. Of course to really nail it you would have to compare it to an absolutely non ketogenic diet, say one supplying a total of 140g/d of carbohydrate. Does carbosis beat a middling carbohydrate mixed diet? You bet.
Oh, the scribbled-in red numbers came from Table 2 of this paper.
Most people in respectable CICO based mainstream nutrition have never heard of carbosis, Walter Kempner, the Rice Diet and have probably never heard of Denise Minger.
But Kevin Hall has. My respect for his knowledge-base and ingenuity is vast. Such a pity it's wasted on constructing props for his bizarre pet theories of weight control.
While the 7.7% of calories as fat in both studies is something which amuses me greatly, I do have to admit it may just be an hysterical accident.
At least I'm up front about my rather pronounced personal biases and rather peculiar sense of humour.
Peter
Labels:
A post not about Walter Kempner
Tuesday, December 04, 2018
An exchange of half bricks
I would guess that everyone is aware of the study by Ebbeling et al, Ludwig's group, looking at the metabolic effect of low carbohydrate diets on total energy expenditure (TEE, all graphs show kcal/d) in the aftermath of weight loss on a conventional diet.
Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial
I'd like to summarise their data using numbers taken from Tables 2 and 3 which, with a little arithmetic, allows me to produce this graph of TEE at various time points. These are as follows: when the subjects walk off the street (Pre on the graph), after a period of semi-starvation on a conventional diet (Start) and then during weight stability on a high, medium or low carbohydrate diet (End). The plot looks like this:
In the study they compared the change from the Start TEE to the End TEE, ie they used these data points:
They took the absolute changes from Start to End thus and got a resultant p of less than 0.05
This, obviously, is completely unacceptable. Well, it is if you are Kevin Hall. So now we have this
No Significant Effect of Dietary Carbohydrate versus Fat on the Reduction in Total Energy Expenditure During Maintenance of Lost Weight
What Ludwig's group did wrong (amongst the many other things pointed out by Hall and Guo) is that they used the wrong data points.
Recall the original graph:
According to Hall: If you want to ask about the effect of low carbohydrate diets on the depression in TEE produced by conventional semi-starvation you should NOT compare the semi-starved TEE (as in Start) to the TEE on a high, medium or low carbohydrate diet (End). You should instead use the TEE expenditure at randomisation (Pre on the graph). Like this:
Using Pre as your anchor point you can draw the same data thus:
Which obviously gives us p greater than 0.05 and all of the benefits of low carbohydrate diets are lost. Phew. Happy Hall. But why should anyone use the Pre values as an anchor point?
Now, no one is an unbiased researcher. Hall is, surprisingly, no exception. Hence the current exchange of half bricks in the BMJ.
As I see it the Ebbeling paper looks at the effect of LC eating on the damage done to TEE by conventional dieting.
What Hall wants the analysis to do instead is to look at the overall effect of damage done to TEE by conventional semi-starvation combined with partial rescue during weight-stable LC eating vs the combined damage done by conventional semi-starvation followed by maintained damage done by HC weight-stable eating. As he writes:
"However, the final analysis plan was modified to make the diet comparisons with the TEE measurements collected in the immediate post-weight loss period rather than at the pre-weight loss baseline"
To me Hall is stating that Ebbeling et al almost did make the "Hall" mistake of using the "Pre" TTE as anchor point but corrected this at the 11th hour, still before blinding was unmasked. What puzzles me is how Ebbeling could have ever even considered using the "pre weight loss baseline" as the anchor point in the original study design.
The massive benefit to Hall of including the conventional semi-starvation active weight loss period along with the intervention weight stability period is to dilute the remedial biological effect of LC eating out of statistical significance.
The core information which the study provides is about the remedial effect of LC eating on correcting the damage done by a conventional semi-starvation period. That effect only happens between "Start" and "End", which is when carbohydrate restriction is applied.
That's one of the MASSIVE problems with carbohydrate restricted eating. It only provides benefit when you don't eat carbohydrate!
Including data from "Pre" right through to "End" dilutes the clearly demonstrable biological effect of carbohydrate restriction on reduced TEE post conventional dieting.
So what doe the title and text of Hall's rebuttal tell us? Either about Hall or about TEE? Don't over think it!
I would also declare that my own biases are a conflict of interest but if you need me to say that then you have probably arrived here by accident, you know where the back button is.
However I would say that I am ambivalent about the importance of the TEE changes, though I suspect they do happen. What really matters to me is what happened in Aberdeen over a decade ago.
Peter
Edit
More excellent half bricks here
End edit.
Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial
I'd like to summarise their data using numbers taken from Tables 2 and 3 which, with a little arithmetic, allows me to produce this graph of TEE at various time points. These are as follows: when the subjects walk off the street (Pre on the graph), after a period of semi-starvation on a conventional diet (Start) and then during weight stability on a high, medium or low carbohydrate diet (End). The plot looks like this:
In the study they compared the change from the Start TEE to the End TEE, ie they used these data points:
They took the absolute changes from Start to End thus and got a resultant p of less than 0.05
This, obviously, is completely unacceptable. Well, it is if you are Kevin Hall. So now we have this
No Significant Effect of Dietary Carbohydrate versus Fat on the Reduction in Total Energy Expenditure During Maintenance of Lost Weight
What Ludwig's group did wrong (amongst the many other things pointed out by Hall and Guo) is that they used the wrong data points.
Recall the original graph:
According to Hall: If you want to ask about the effect of low carbohydrate diets on the depression in TEE produced by conventional semi-starvation you should NOT compare the semi-starved TEE (as in Start) to the TEE on a high, medium or low carbohydrate diet (End). You should instead use the TEE expenditure at randomisation (Pre on the graph). Like this:
Using Pre as your anchor point you can draw the same data thus:
Which obviously gives us p greater than 0.05 and all of the benefits of low carbohydrate diets are lost. Phew. Happy Hall. But why should anyone use the Pre values as an anchor point?
Now, no one is an unbiased researcher. Hall is, surprisingly, no exception. Hence the current exchange of half bricks in the BMJ.
As I see it the Ebbeling paper looks at the effect of LC eating on the damage done to TEE by conventional dieting.
What Hall wants the analysis to do instead is to look at the overall effect of damage done to TEE by conventional semi-starvation combined with partial rescue during weight-stable LC eating vs the combined damage done by conventional semi-starvation followed by maintained damage done by HC weight-stable eating. As he writes:
"However, the final analysis plan was modified to make the diet comparisons with the TEE measurements collected in the immediate post-weight loss period rather than at the pre-weight loss baseline"
To me Hall is stating that Ebbeling et al almost did make the "Hall" mistake of using the "Pre" TTE as anchor point but corrected this at the 11th hour, still before blinding was unmasked. What puzzles me is how Ebbeling could have ever even considered using the "pre weight loss baseline" as the anchor point in the original study design.
The massive benefit to Hall of including the conventional semi-starvation active weight loss period along with the intervention weight stability period is to dilute the remedial biological effect of LC eating out of statistical significance.
The core information which the study provides is about the remedial effect of LC eating on correcting the damage done by a conventional semi-starvation period. That effect only happens between "Start" and "End", which is when carbohydrate restriction is applied.
That's one of the MASSIVE problems with carbohydrate restricted eating. It only provides benefit when you don't eat carbohydrate!
Including data from "Pre" right through to "End" dilutes the clearly demonstrable biological effect of carbohydrate restriction on reduced TEE post conventional dieting.
So what doe the title and text of Hall's rebuttal tell us? Either about Hall or about TEE? Don't over think it!
I would also declare that my own biases are a conflict of interest but if you need me to say that then you have probably arrived here by accident, you know where the back button is.
However I would say that I am ambivalent about the importance of the TEE changes, though I suspect they do happen. What really matters to me is what happened in Aberdeen over a decade ago.
Peter
Edit
More excellent half bricks here
End edit.
Subscribe to:
Posts (Atom)