Here is a schematic illustration of the generation of lactate from glucose as suggested by Schurr, ready for doodling on:
In more artistic style, from Figure 1 in Schurr's paper, it looks like this:
If we look at the more traditional view of glycolysis to pyruvate with a deficit of NAD+ which must be made up by the mtG3Pdh enzyme we have something which looks quite like this:
The concept here is that mtG3Pdh is being used to regenerate the NAD+ which is essential for glycolysis to proceed to pyruvate. Of course, this concept becomes obsolete as soon as we adopt the lactate hypothesis. I tend to view reality as a combination of two pathways like this:
Normal function is via lactate with NAD+ and NADH in perfect balance. The generation of NAD+ from NADH by mtG3Pdh reduces the redox potential of the cytoplasm so the drive to generate lactate, while NADH is in short supply, becomes unhelpful or frankly impossible. For each NADH utilised by mtG3Pdh it is necessary to abort glycolysis at pyruvate, saving the one equivalent NADH by terminating it here rather than at lactate.
It's not that mtG3Pdh replenishes essential NAD+, it's that its consumption of NADH obliges termination of glycolysis at pyruvate. So is mtG3Pdh doing, if the traditional view is a load of bollocks?
Insulin signalling:
Under conditions of high glucose throughput, with insulin signalling, glycolysis will terminate at pyruvate in proportion to the activation of the glycerophosphate shuttle. Notice the two blue arrows signifying two actions of insulin. If we go back to Veech once more, with his isolated heart preparations, we have these two very specific actions of insulin:
One is an increase in glycogen synthesis. Flooding cardiac myocytes with insulin puts every GLUT4 possible on to the cell surface and intracellular glucose approaches that of the perfusing buffer, in this case 10mmol/l. The cells have absolutely no use for this much suddenly available glucose and the simple solution is to divert it to glycogen. The use of intensive insulin therapy in humans with T2D always improves non-oxidative glucose disposal. It goes to glycogen. They get fat too of course. I guess we could quote Veech's abstract:
"The administration of saturating doses of insulin to the glucose perfused, working rat heart acutely increased activity of the glucose transporter 4, GLUT 4, in the plasma membrane (equilibrating extracellular glucose and intracellular [glucose]), activated glycogen synthase (stimulating the rate of glycogen synthesis), and increased mitochondrial acetyl CoA production by the pyruvate
dehydrogenase multienzyme complex. Unexpectedly, insulin increased cardiac hydraulic work but decreased net glycolytic flux and O2 consumption, improving net cardiac efficiency by 28%".
The second arrow points to the mitochondria. Insulin acts to produce a covalent change in the ETC proteins which improves ATP generation per unit oxygen consumed.
In Veech's isolated rat heart preparation insulin REDUCES glycolysis, increases glycogen synthesis and increases mitochondrial ETC efficiency. It's all in the paper on the link above and in assorted related papers, many of which are free full texts and cover the basics. And not so basics.
Veech was looking at combinations of glucose, insulin and ketone bodies. Unlike myself, he is no lover of fatty acids. Ah well. So the buffer used had no fatty acids at all. Now, what do we think insulin will do to fatty acids? It has already maximised the efficiency of the ETC and shunted excess glucose to (harmless) glycogen.
Does insulin facilitate or inhibit the oxidation of fatty acids? The process is just like the diversion of glucose to glycogen:
Once upon a time, before insulin kicked in, CPT1 was used to supply fatty acids to the mitochondria for beta oxidation. Given the increased efficiency of ox phos under insulin, the fate of a significant proportion of fatty acids presenting for beta oxidation is to meet a blockade at CPT1 and to be diverted towards re esterification to triglycerides in the cytoplasm.
I wouldn't suggest that intramuscular triglycerides cause insulin resistance per se. But derivatives there-of do appear to do so and there is certainly an association between intramyocyte triglyceride accumulation and insulin resistance, particularly in non athletes.
This all well and good. Things become more interesting when we take a mtG3Pdh knockout mouse, which cannot signal through mtG3Pdh, and make it run. And run. And run. Until exhaustion, whenever that might be. Of course we also have a rather popular drug to inhibit mtG3Pdh which might mimic the knockout situation. Perhaps for the next post.
Peter
Wednesday, October 28, 2015
Wednesday, October 14, 2015
Protons (37) full glycerophosphate shuttle knockout mice
Just a short post on this paper:
Lethal Hypoglycemic Ketosis and Glyceroluria in Mice Lacking Both the Mitochondrial and the Cytosolic Glycerol Phosphate Dehydrogenases
The glycerol-3-phosphate shuttle is composed of a cytosolic G3Pdh which actually hydrogenates dihydroxy acetone phosphate to glycerol-3-phosphate, using NADH, and a second, mitochondrial version, which does the actual dehydrogenating reconversion back to dihydroxy acetone phosphate. Assuming things are going in the most usual direction.
Lab models of mice with knockout of either part of the glycerol-3-phosphate shuttle are very interesting and are long term survivors, to be discussed another day. Today I’d just like to think about those mice with a complete knockout, deleting both components. They die at a few days of age with an array of problems, lethal hypoglycaemia being the end crisis.
At this stage of life they are being fed a very high fat, low carbohydrate diet (mouse milk, it's mostly palmitic acid. That should tell you something!) and they are very heavily reliant on gluconeogensis to maintain adequate glucose levels using glycerol from the milk's triglycerides as the necessary glucose precursor. As the paper says:
“Glyceride-glycerol is an especially important gluconeogenic precursor in the neonatal mouse, because 80% of calories from mouse milk are derived from fat, 16–17% from protein, and only 2–5% from lactose (20, 22–24). Thus total calories available from dietary glycerol ( 4%) equal calories from lactose.”
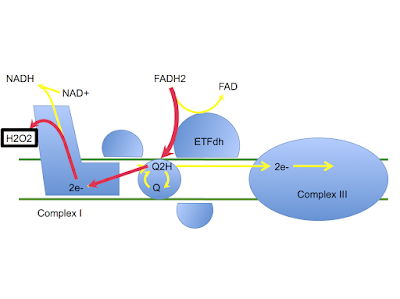
Now, if you are a lab mouse drinking a low carbohydrate diet, should you be insulin sensitive or insulin resistant? If you are insulin sensitive, how much glucose would you like to waste in your muscles, when you only have a limited supply of the stuff in the first place? I would like to suggest that, even in mice, burning glucose for fuel when there is a very limited supply, might not be a survival trait. So insulin resistance, physiological, might be essential for survival. If physiological resistance is essential this is why they have evolved to produce palmitic acid as the main fat in the maternal milk supply. What is needed is a decent input at ETFdh plus and some input at mtG3Pdh to drive enough electrons backwards through complex I to achieve full physiological insulin resistance.
These mice have zero input at mtG3Pdh so are reliant on ETFdh working under palmitic acid to try and achieve this. This clearly doesn't hack it.
The electron transport chain has an ad libitum supply of palmitic acid. The question is:
Without the glycerophosphate shuttle, can the ETC generate reverse electron flow using ETFdh alone to trigger enough superoxide to adequately resist insulin's wasteful usage of precious glucose?
Obviously not. So the second question has to be:
Can pamitic acid at levels in excess of 900micromol/l generate just enough superoxide to allow insulin signalling to commence, without assistance from mtG3Pdh?
I suspect the answer is yes. This generates just enough superoxide (hence H2O2) to allow insulin signalling to commence. Active but inappropriate insulin signalling then triggers a fatal fall in blood glucose as GLUT4s translocate to cell surfaces and glucose drops through them to be squandered irreplaceably. Glucose crashes, the mice die.
It's an extreme model but it makes us think about what might be happening in addition the malonyl-CoA and CPT1 level of signalling and the limited gluconeogenesis discussed so very nicely in the paper.
Peter
Lethal Hypoglycemic Ketosis and Glyceroluria in Mice Lacking Both the Mitochondrial and the Cytosolic Glycerol Phosphate Dehydrogenases
The glycerol-3-phosphate shuttle is composed of a cytosolic G3Pdh which actually hydrogenates dihydroxy acetone phosphate to glycerol-3-phosphate, using NADH, and a second, mitochondrial version, which does the actual dehydrogenating reconversion back to dihydroxy acetone phosphate. Assuming things are going in the most usual direction.
Lab models of mice with knockout of either part of the glycerol-3-phosphate shuttle are very interesting and are long term survivors, to be discussed another day. Today I’d just like to think about those mice with a complete knockout, deleting both components. They die at a few days of age with an array of problems, lethal hypoglycaemia being the end crisis.
At this stage of life they are being fed a very high fat, low carbohydrate diet (mouse milk, it's mostly palmitic acid. That should tell you something!) and they are very heavily reliant on gluconeogensis to maintain adequate glucose levels using glycerol from the milk's triglycerides as the necessary glucose precursor. As the paper says:
“Glyceride-glycerol is an especially important gluconeogenic precursor in the neonatal mouse, because 80% of calories from mouse milk are derived from fat, 16–17% from protein, and only 2–5% from lactose (20, 22–24). Thus total calories available from dietary glycerol ( 4%) equal calories from lactose.”
Now, if you are a lab mouse drinking a low carbohydrate diet, should you be insulin sensitive or insulin resistant? If you are insulin sensitive, how much glucose would you like to waste in your muscles, when you only have a limited supply of the stuff in the first place? I would like to suggest that, even in mice, burning glucose for fuel when there is a very limited supply, might not be a survival trait. So insulin resistance, physiological, might be essential for survival. If physiological resistance is essential this is why they have evolved to produce palmitic acid as the main fat in the maternal milk supply. What is needed is a decent input at ETFdh plus and some input at mtG3Pdh to drive enough electrons backwards through complex I to achieve full physiological insulin resistance.
These mice have zero input at mtG3Pdh so are reliant on ETFdh working under palmitic acid to try and achieve this. This clearly doesn't hack it.
The electron transport chain has an ad libitum supply of palmitic acid. The question is:
Without the glycerophosphate shuttle, can the ETC generate reverse electron flow using ETFdh alone to trigger enough superoxide to adequately resist insulin's wasteful usage of precious glucose?
Obviously not. So the second question has to be:
Can pamitic acid at levels in excess of 900micromol/l generate just enough superoxide to allow insulin signalling to commence, without assistance from mtG3Pdh?
I suspect the answer is yes. This generates just enough superoxide (hence H2O2) to allow insulin signalling to commence. Active but inappropriate insulin signalling then triggers a fatal fall in blood glucose as GLUT4s translocate to cell surfaces and glucose drops through them to be squandered irreplaceably. Glucose crashes, the mice die.
It's an extreme model but it makes us think about what might be happening in addition the malonyl-CoA and CPT1 level of signalling and the limited gluconeogenesis discussed so very nicely in the paper.
Peter
Saturday, October 10, 2015
Protons (38) and ultra low fat once more
OMG, here we go with more doodles. Ah well...
This post is a summary of the ideas which came out of the Protons thread. I’ve been meaning to write it for some time but the trigger was really Denise Minger’s idea of “carbosis”. This has made me revisit ideas I kicked around at the time of the Potato Diet about the systemic level of insulin and revisited within the Protons concept. I guess the idea is to try and work out whether there is any physiologically plausible explanation for the changes seen under ultra low fat eating, under carbosis.
This is my opinion, most of the references are buried in the Protons thread and some ideas I have interpolated from hard facts because they make sense to me. But be aware you are reading an opinion piece.
Cellular insulin response is controlled by superoxide produced at complex I, which exits the mitochondria as H2O2. Small pulses of H2O2 limit the activity of PTP 1B (protein tyrosine phosphatase 1B). Disabling PTP 1B takes the brakes off of the insulin receptor and allows it to autophosphorylate whenever insulin binds and so allows subsequent insulin signalling to take place.
Large amounts of H2O2 inhibit the autophosphorylation of the insulin receptor directly, at several sites, and so cause insulin resistance per se.
So it's pretty obvious that mitochondrial superoxide/H2O2 controls insulin function and subsequent blood glucose levels, and obesity levels if you are a True Believer, which I am. In this post I'd just like to summarise my own personal thoughts on how the electron transport chain, from where much of the the superoxide is produced, behaves under a variety of conditions.
It is quite easy to set up a mitochondrial preparation which can be driven almost completely through complex I. You can feed it on pyruvate/malate. Under these conditions there is essentially zero H2O2 generation, so there is obviously very limited superoxide generation. Think of it like this:
Electrons from NADH simply drop through complex I, fall easily down hill on to the CoQ couple and are handed on to complex III and the rest of the chain. Similarly we can run tissues on pure glucose at modest levels, even if that's not quite how we do it in real life. Many of Veech's papers on ketones used isolated heart preparations which work quite well for quite some time on oxygenated buffer with glucose alone as the sole metabolic substrate, without insulin. So mitochondria can work on pyruvate and isolated hearts can work on pure glucose.
The next piece of input we need to consider is succinate dehydrogenase (SDH), also known as complex II but I'll use SDH as the term in this post. As the TCA turns using one molecule of acetyl-CoA it generates 3 molecules of NADH and one of FADH2, the later being embedded deep within SDH. The FADH2 of succinate dehydrogenase feeds directly to the CoQ couple and reduces it independently of electrons coming from complex I. We have a situation like this:
The TCA is turning and the ETC is accepting input at two points. This is normal physiology and generates very little superoxide, especially while rest of the ETC is well oxidised and so very willing to accept electrons.
With a mitochondrial preparation it is very easy to supply an input of exogenous succinate to SDH without the rest of the TCA cycling in synchrony. This is an experimental situation, only seen in in-tact animals if they are dosed orally with succinc acid esters. This is what happens:
The diagram doesn't make it terribly clear so lets clarify the flow of electrons from SDH, given a sudden massive rise in isolated succinate. Reverse electron flow to superoxide is picked out in red:
Feeding high levels of succinate to a mitochondrial preparation produces massive levels of superoxide. This is not physiology but it can be viewed as a pharmacological demonstration of physiology pushed beyond its normal limits. It's an illustration.
Aside: mitochondria normally oscillate in their function. The TCA drives complex I via NADH (mostly from the early part of the TCA) to the CoQ couple. SDH reduces the CoQ couple independently so opposes this process. Oxaloacetate at the end of the TCA inhibits SDH, facilitating input at complex I, and the whole system goes back and forth as a normal oscillatory process. That's how it is. I’ve no idea why it’s organised this way, but it clearly works rather well! End aside.
But we can say that some degree of reverse electron flow from SDH through complex I will allow small amounts of superoxide generation which will generate modest pulses of H2O2 as far as PTP 1B. This is essential for insulin signalling.
I feel the next step is to look at the situation where glucose is in oversupply, classically after a pure starch meal. Here we need a brake to be applied to the influx of glucose to the cell. In the aftermath of a glucose based meal we would expect insulin to be high, GLUT4s to be active and ox phos to be based on pyruvate (or lactate if you prefer). What is needed is to reduce insulin signalling, limit GLUT4 translocation and so limit glucose ingress to that which is needed by the cell. This is achieved through a side branch of the glycolytic pathway which generates glycerol-3-phosphate. Among the many, many functions of G-3-P, one is to input electrons from NADH to the electron transport chain from the cytoplasmic side of the inner mitochondrial membrane which will reduce the CoQ couple. How much it does this probably depends on the level of metabolites running down through glycolysis. Let's think about a high glycolytic flux, marked reduction of the CoQ couple and see what happens:
The things to note are a large input of cytoplasmic NADH generating marked reverse electron flow through complex I to generate enough superoxide to send H2O2 to inhibit the action of the insulin/receptor complex. It's a simple negative feedback situation and probably produces quite precise control of access of glucose tailored to the needs of the individual cell. There is no pathology here, it's how glucose based metabolism should be controlled.
The next scenario to consider is something like a large ingress of uncontrollable carbohydrate. A find of honey or table sugar. Let's think about it in the complete absence of any fatty acid metabolism. If we have a sudden avalanche of fructose which pours down through glycolysis, what happens? This unstoppable cascade will activate mtG3Pdh much as the excess glucose we have just considered. This should produce a large amount of H2O2 and disable activity of the insulin receptor and produce enough limitation of GLUT4 translocation to exactly limit glucose access by the correct amount to offset the fructose flood. There are other issues from fructose but these are asides. If metabolism is based on pure glycolysis to supply ox phos substrate then fructose can be accommodated by reducing glucose ingress. From the cellular point of view it all balances out and the degree of insulin resistance is at appropriate physiological levels and only occurs while fructose is high, i.e. not for very long. I'll leave uric acid and metabolic syndrome out from the current discussion, needless to say there are issues, for and against.
So I view mtG3Pdh as a balancing act controlling access of carbohydrate to ox phos by controlling insulin signalling through reverse electron transport through complex I. If these are the only components supplying the ETC it seems to be a pretty simple balancing act which might work rather well. On a pure glucose diet some fructose, even quite a lot, is no problem.
Now we have to add in free fatty acids. While beta oxidation generates acetyl CoA and NADH, one molecule of each for each pair of carbon atoms in the chain, they also generate a molecule of FADH2 at the same time. FADH2 is never used as an unbound molecule, here it is stored within electron transporting flavoprotein which delivers FADH2's electrons to the ETC at electron transporting flavoprotein dehydrogenase (ETFdh in the diagram). Much the same as glycerol-3-phosphate at mtG3Pdh, ETFdh reduces the CoQ couple and is adept at driving reverse electron flow through complex I. Low inputs will do nothing or merely generate small pulses of H2O2 at activating levels, high inputs will generate high levels of H2O2 to shut down insulin signalling on the basis that there is plenty of metabolic substrate from fats, minimal glucose is needed, thank you very much. Again, this is pure physiology, I see no pathology in it.
It looks like this:
The step in beta oxidation which produces the FADH2 to drive ETFdh does not occur when the fatty acid being processed presents a double bond at this step. So fully saturated fatty acids generate the maximum amount of FADH2, monounsaturated fats somewhat less and PUFA least of all. The exception is any fatty acids longer than about 18 carbon atoms, these go to peroxisomes rather than mitochondria. So if we go on to consider monounsaturated FFAs we have something like this:
MUFAs produce significantly less FADH2 so less reduction of the CoQ couple and are not used to generate nearly as much insulin resistance as fully saturated fatty acids. This is not surprising as MUFAs are desaturated versions of fully saturated fats and the desaturation process is largely activated by insulin. MUFAs can be thought of as a more carbohydrate tolerant version of saturated fats.
Of course by the time we get to linoleic acid there is even less FADH2 generated and we have very little ability to resist insulin when these are the main fatty acids being oxidised. Omega 6 PUFA facilitate the action of insulin but don't suppress it even when being metabolised in bulk:
So we can view a glucose system in balance with a fatty acid system where the input to the CoQ couple from the fatty acids controls insulin sensitivity to meter glucose access through manipulating insulin signalling or lack there-of.
Saturated fats suit low glucose availability, MUFA suit a mixed diet and PUFA are spawn of the devil. Near zero fatty acids in the mix rely on mtG3Pdh to regulate glycolysis flux.
I suppose we also ought to think of the situation under a large, uncontrolled fructose input through mtG3Pdh occurring at the same time as saturated fatty acids are being oxidised. That gives us this scenario:
Having two inputs reducing the CoQ couple (as well as a little input from SDH) is a perfect recipe for driving extreme reverse electron transport through complex I with the production of completely unreasonable quantities of superoxide and H2O2. This is the scenario of free radical mediated damage combined with serious insulin resistance. D12079B anyone? The problems are less severe with PUFA fats but this leaves us with a different set of problems, not for today. OK.
Before we go on to look at more human based scenarios using low fat diets I guess we need to consider insulin secretion by the pancreas. This is not solely controlled by glucose. In fact I think I’m going to include the only reference in this post as I don’t think I’ve cited this particular paper before, though I may have done so and forgotten!
Suppress most FFAs using nicotinic acid and you will completely abort the secretion of insulin generated by a glucose level of 12.5mmol/l. Replace the FFAs by infusion and you find that insulin secretion is markedly affected by the nature of the FFAs. The longer the acyl chain the more insulin is secreted. The more double bonds, the less insulin is secreted. At a FFA level around 0.1mmol/l, acutely induced, the pancreas will not secrete insulin in response to 12.5mmol/l of glucose. It seems to me that getting FFAs this low is well within the realms of possibility using a near zero fat diet. You are then in to the region of minimal insulin secretion combined with maximal insulin sensitivity. I know it’s a rat model, a perfused pancreas, assorted artificial lipid infusions, but the logic holds well. Insulin secretion and insulin response/resistance are remarkably similar processes.
So hypo insulinaemia with marked insulin sensitivity might well be the hallmarks of carbosis. I won’t reiterate my thoughts about hepatic insulin extraction except I see this as complementary to the reduced secretion under ultra low fat conditions.
As a True Believer I cannot see steady weight loss without reduced systemic insulin levels. Carbosis suggests that this is the real situation.
The phrase is ITIS, we all know what this stands for. Low enough fat can quite simply cripple the pancreas' ability to secrete insulin in response to glucose. Low enough fat and there is very limited ability to generate superoxide levels beyond normal PTP 1B inhibiting levels. If we accept that hyperinsulinaemia is the driving force of metabolic syndrome and all of its sequelae we have, under conditions of extreme fat restriction, the potential for reducing insulin while using a maximal carbohydrate diet. i.e. There is a health benefit to carbosis, possibly major, implausible as it seems.
There. I said it. You cannot argue with the physiology.
I believe this is what Denise Minger might be describing using "carbosis" as the corollary of ketosis. Under both of these conditions there is minimal insulin secretion but under carbosis there is enough insulin sensitivity working through mtG3Pdh to accurately regulate a near pure glucose metabolism. Fructose is no problem as there is plenty of "exchangeable" glucose for use in a substitution manner. Fatty acids have to be very low for "carbosis" to occur at all and it will be degraded far more easily by saturated fats than by PUFA, as per the ETC diagrams above and as per Denise’s examples.
The essential feature is low insulin. This is the commonality with ketosis. If there are going to be any health benefits of carbosis the low insulin will be the driving force.
So, am I a convert? This is not a religion, what I would ask is:
How effective is carbosis in the real world of T2 diabetes?
As Denise comments:
"More than half of those 100 diabetic ricers—63%—actually saw their fasting blood sugar drop by at least 20 mg/dL during the diet. Only 15% had their blood sugar go up significantly. The remaining 22 saw little to no change".
Translation: Blood glucose: 15% of people were f*cked. 22% it didn’t help. 63% could maintain carbosis.
Insulin usage:
"‘Twas a similar story in Insulin Land. Of the study’s participants, 68 entered the scene already dependent on insulin. As the carbs raged on, 21 of those insulin-injecters didn’t have to change their dosage; nine needed an increase (including four people who initially weren’t on any insulin at all); and—again comes the cruel, cruel defiance of prediction—42 slashed their usage significantly. In fact, 18 folks were able to discontinue their insulin entirely. Feasting on white rice. And sugar. And fruit juice".
Translation: Insulin usage: 13% were f*cked. 29% derived no benefit. 58% achieved carbosis.
How does this stack up against rather mild carbohydrate restriction in severe T2 diabetics?
This diagram says it all. It's from Haimoto et al in 2009.
All they did was drop carbohydrate intake to just over 130g/d. No ketosis. Look at the changes for the first 3 months in HbA1c:
No one needed to increase meds. No one failed to drop HbA1c. No one had to start on insulin. Most people dropped their sulpha drugs. The large spike upwards in the second section looks like one of the two drop outs. The other drop out seems to be lost in the variation in maintenance of control over the 3-6 month interval. Bear in mind that 130g/d is a VERY modest approach to low carbohydrate dieting in severe T2 diabetes. No ketosis, just 100% response rate to a modest carbohydrate reduction.
How can you compare carbosis with ketosis, or even mild carbohydrate restriction? It's like comparing boiled rice followed by boiled rice plus table sugar with a char-grilled fatty steak (rib eye is my preferred choice), buttered broccoli on the side plus Optimal ice-cream to follow. With extra double cream if you're losing too much weight.
The biochemistry of carbosis is very interesting. It might help just over a half of people who try it. Its therapeutic use seems to be of dubious relevance when real food can provide results in 100% of people who comply to carbohydrate reduction. It's strictly for the anhedonic out there but even these poor souls should be cautious about finding themselves in the group of 13-15% who end up f*cked, metabolically speaking.
No thanks.
Peter
Tuesday, October 06, 2015
Meet the researchers with the PKCζ deletion
Let's get this straight, right at the start: If you have cancer your outlook is probably worse if that cancer cell population has lost its ability to produce the metabolic regulator PKCζ. It's a bad news deletion.
We are looking at this paper (via David Ramsay, I think):
Control of Nutrient Stress-Induced Metabolic Reprogramming by PKCζ in Tumorigenesis
If you were remotely considering a ketogenic diet as a therapy for cancer management I think you might rightly be interested in the paper. Unfortunately it's quite technical (TL;DR perhaps Heather Buschman?) so perhaps it might be easier to go to the article in ScienceDaily for the take-home message. So initially let's look at the press release, the summary from which says this:
"Many scientists have tried killing tumors by taking away their favorite food, a sugar called glucose. Unfortunately, this treatment approach not only fails to work, it backfires--glucose-starved tumors get more aggressive. In a new study, researchers discovered that the protein PKCζ is responsible for this paradox. The research suggests that glucose depletion therapies might work, as long as the cancer cells produce PKCζ"
There are some pretty sweeping and unsupported statements here. If you actually read the paper itself you will find that there is a mass of information derived from cell culture under extreme (zero glucose, high glutamate) conditions which, while it does allow picking out the details of why PKCζ deletion might be so bad, tells you little about real life progression of cancer.
The group made two attempts to transfer their information from cell culture to something resembling real life. One approach was that they investigated, observationally, humans with colorectal cancer.
They found that in these people the outlook is worse if their particular cancer is PKCζ negative. Fair enough. However, attempted glucose restriction applied to humans with colorectal cancer is not exactly a widespread practice and was not mentioned in these patients. So it seems to be a reasonable assumption that those folks with PKCζ negative cancers are dying at an accelerated rate under glucose replete conditions, not under the conditions used in cell culture experiments. But we don't know for sure, there is no information about the blood chemistry or nutritional management of the patients.
The epic fail in the paper comes with the obligatory mouse model, tacked on near the end.
All you have to do is to mate cancer prone mice with mice which are PKCζ negative and a proportion of their offspring will be cancer prone and either PKCζ positive or negative. You can then test your hypothesis that glucose restriction promotes aggressive growth in PKCζ negative cancer prone mice, whose cancers will clearly be PKCζ negative too.
This should be easy.
Just feed the PKCζ negative mice something like that lovely F3666 ketogenic diet and compare them to those fed standard crapinabag. Obviously the low glucose levels from the ketogenic diet will promote early death in the PKCζ negative group as metabolism switches from glucose to glutamine and the cells develop an aggressive phenotype. The cell cultures say this will happen.
But they didn't do this. They actually fed D12079B vs crapinabag.
What is D12079B, you may ask, that you would use it to show that glucose restriction is Badness for PKCζ negative cancer victims?
D12079B is a typical Western Diet, sucrose/butter derived and is specifically marketed to produce metabolic syndrome in mice. Hyperglycaemia with hyperinsulinaemia. The exact, absolute, reliable opposite of the zero glucose used in all of the cell culture work.
Under glucose excess the animals with PKCζ knockout SHOULD have done at least as well as those on crapinabag, because all the cell culture work showed excess cancer growth during glucose RESTRICTION.
But this didn't happen. Under glucose excess the PKCζ knockouts died fastest of all the groups examined.
Let's just emphasise: At no point did the researchers attempt to limit glucose supply in anything even remotely resembling a live animal.
Does glucose restriction promote and aggressive cancer phenotype through a switch to glutamine metabolism, outside of cell culture?
No one knows. Certainly not the authors of the paper and don't get me started on the press release scribbler.
Personally I'm cautious about ketogenic diets in cancer. I'd expect them to do some good but only time will tell how much good and in which cancers.
But I can see a cop-out in a research paper a mile off. Did they try a few mice on F3666 and fail to report it because F3666 was protective? Or did they simply not dare test their hypothesis? At some stage there was a meeting of the senior researchers where the (extremely expensive) transition to a mouse model was discussed. They did not just stick a needle in a list of diets to choose D12079B. Years of work suggested looking at glucose restriction. They deliberately did the opposite. I shake my head in disbelief.
Peter
We are looking at this paper (via David Ramsay, I think):
Control of Nutrient Stress-Induced Metabolic Reprogramming by PKCζ in Tumorigenesis
If you were remotely considering a ketogenic diet as a therapy for cancer management I think you might rightly be interested in the paper. Unfortunately it's quite technical (TL;DR perhaps Heather Buschman?) so perhaps it might be easier to go to the article in ScienceDaily for the take-home message. So initially let's look at the press release, the summary from which says this:
"Many scientists have tried killing tumors by taking away their favorite food, a sugar called glucose. Unfortunately, this treatment approach not only fails to work, it backfires--glucose-starved tumors get more aggressive. In a new study, researchers discovered that the protein PKCζ is responsible for this paradox. The research suggests that glucose depletion therapies might work, as long as the cancer cells produce PKCζ"
There are some pretty sweeping and unsupported statements here. If you actually read the paper itself you will find that there is a mass of information derived from cell culture under extreme (zero glucose, high glutamate) conditions which, while it does allow picking out the details of why PKCζ deletion might be so bad, tells you little about real life progression of cancer.
The group made two attempts to transfer their information from cell culture to something resembling real life. One approach was that they investigated, observationally, humans with colorectal cancer.
They found that in these people the outlook is worse if their particular cancer is PKCζ negative. Fair enough. However, attempted glucose restriction applied to humans with colorectal cancer is not exactly a widespread practice and was not mentioned in these patients. So it seems to be a reasonable assumption that those folks with PKCζ negative cancers are dying at an accelerated rate under glucose replete conditions, not under the conditions used in cell culture experiments. But we don't know for sure, there is no information about the blood chemistry or nutritional management of the patients.
The epic fail in the paper comes with the obligatory mouse model, tacked on near the end.
All you have to do is to mate cancer prone mice with mice which are PKCζ negative and a proportion of their offspring will be cancer prone and either PKCζ positive or negative. You can then test your hypothesis that glucose restriction promotes aggressive growth in PKCζ negative cancer prone mice, whose cancers will clearly be PKCζ negative too.
This should be easy.
Just feed the PKCζ negative mice something like that lovely F3666 ketogenic diet and compare them to those fed standard crapinabag. Obviously the low glucose levels from the ketogenic diet will promote early death in the PKCζ negative group as metabolism switches from glucose to glutamine and the cells develop an aggressive phenotype. The cell cultures say this will happen.
But they didn't do this. They actually fed D12079B vs crapinabag.
What is D12079B, you may ask, that you would use it to show that glucose restriction is Badness for PKCζ negative cancer victims?
D12079B is a typical Western Diet, sucrose/butter derived and is specifically marketed to produce metabolic syndrome in mice. Hyperglycaemia with hyperinsulinaemia. The exact, absolute, reliable opposite of the zero glucose used in all of the cell culture work.
Under glucose excess the animals with PKCζ knockout SHOULD have done at least as well as those on crapinabag, because all the cell culture work showed excess cancer growth during glucose RESTRICTION.
But this didn't happen. Under glucose excess the PKCζ knockouts died fastest of all the groups examined.
Let's just emphasise: At no point did the researchers attempt to limit glucose supply in anything even remotely resembling a live animal.
Does glucose restriction promote and aggressive cancer phenotype through a switch to glutamine metabolism, outside of cell culture?
No one knows. Certainly not the authors of the paper and don't get me started on the press release scribbler.
Personally I'm cautious about ketogenic diets in cancer. I'd expect them to do some good but only time will tell how much good and in which cancers.
But I can see a cop-out in a research paper a mile off. Did they try a few mice on F3666 and fail to report it because F3666 was protective? Or did they simply not dare test their hypothesis? At some stage there was a meeting of the senior researchers where the (extremely expensive) transition to a mouse model was discussed. They did not just stick a needle in a list of diets to choose D12079B. Years of work suggested looking at glucose restriction. They deliberately did the opposite. I shake my head in disbelief.
Peter
Subscribe to:
Posts (Atom)