I keep trying to get back to simple things like weight loss/gain/insulin/LA but the electrochemistry won't leave me alone.
Someone, possibly Jaromir (mct4health) or Brad Marshall, pointed out that the pyruvate dehydrogenase complex (PDC) is a significant source of ROS.
So I was rootling around through papers on PDC and read, in a now-lost paper, that the decarboxylase component of the complex converted pyruvate to acetate but that a similar effect could be achieved by reacting isolated pyruvate with a source of free radicals to give acetate and carbon dioxide. It was probably H2O2 they were working with. The decarboxylation is a lot quicker with the enzyme but a chemical source of ROS will get the job done.
Anyway, the significance only dawned on me weeks later and I hadn't saved the paper. It's gone.
There are, it turns out, many papers looking at pyruvate decarboxylation using H2O2, other ROS or RNS. It's a generic trait. I went through this first paper in some detail:
Here's a simplified version of their Figure 1 which is the basic reaction. An electron from the H2O2 attacks the alpha carbon of the pyruvate to give the completely unstable 2-hydroperoxy-2-hydroxypropanoate which spontaneously degrades to acetate and CO2:
"The reaction of pyruvate and H2O2 produces acetate, carbon dioxide (CO2) and water; its transition intermediate has been recently confirmed..."
Essentially the H2O2 is providing an electron which destabilises the alpha carbonyl group and the molecule then rearranges itself in to the decarboxylation products.
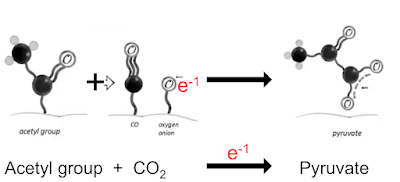
Now look at Nick Lane's slides in the last post. First we need this bit:
in which CO2 accepts a geochemical derived electron to become a bound CO molecule and a bound oxygen anion. This lets us re write this line
(in which the activated CO2 derivatives are highlighted with red ovals) in to the much simpler form of:
Ultimately we can convert an acetyl group and CO2 to pyruvate using a geochemical electron from the origin of life scenario.
We can do the exact opposite and convert pyruvate to acetate and CO2, again using a donated electron, this time from H2O2.
Pyruvate is stable. You can buy it in tablet form as a metabolic nutritional "supplement". It won't convert to vinegar in the jar. If you were to carbonate a bottle of vinegar it would stay as "fizzy vinegar" long term without converting to pyruvate. Exactly as you could mix H2 and CO2 in aqueous solution and they would remain stable without a hint of formate formation. Until you add an electron.
Then the reaction moves. The change in energy is quite small and you could push the reaction one way or the other way depending on the relative concentrations of acetate, CO2 or pyruvate. The electron is what makes it happen, in either direction.
This is not unique to the reaction of pyruvate with hydrogen peroxide.
A little more grubbing around suggests that peroxinitrite is even better:
"... oxygen consumption studies confirmed that peroxynitrite mediates the decarboxylation of pyruvate to free radical intermediates. Comparing the yields of acetate and free radicals estimated from the oxygen uptake studies, it is concluded that pyruvate is oxidized by both one- and two-electron oxidation pathways..."
No one seems to have looked at superoxide but you can bet your bottom dollar it does the same. In fact, for the similar reaction of alpha ketoglutarate to succinate, superoxide will do the job:
The Tricarboxylic Acid Cycle, an Ancient Metabolic Network with a Novel Twist
"The significance of KG [alpha ketoglutarate], a metabolite that can detoxify H2O2 and O2- with the concomitant formation of succinate in this process is also discussed."
"The significance of KG [alpha ketoglutarate], a metabolite that can detoxify H2O2 and O2- with the concomitant formation of succinate in this process is also discussed."
So what?
The interconversion of metabolites of the TCA appears to be quite possible mediated by nothing other than the availability of spare electrons and the relative concentrations of the core reactants. From Nick Lane's doodles the essential component for the actual dissipation of energy in the sections of the TCA at the origin of life are actually mediated by the availability of electrons. No enzymes required.
The availability of electrons from geochemical sources is what drove the conversion of CO2 and protons to core metabolites of the essential parts of the TCA. No electrons, no conversion of anything in to anything else.
Today the electrons come from all sorts of places, reduced ferredoxin, NADH, FADH2 etc. But originally, in the beginning, I suspect that the first source of free electrons to replace the geochemical source might have been superoxide.
We know that LUCA used oxygen despite the anoxic conditions of the early Earth. She had superoxide dismutase, catalase and a precursor of haemoglobin which stored (precious) oxygen. So LUCA actively controlled oxygen availability, superoxide dismutation and hydrogen peroxide catabolism to oxygen and water. Presumably for a very specific purpose.
All that is needed to convert pyruvate to acetate is a free electron. Electrons are continuously being placed on to ferredoxin by our prototypical membrane bound hydrogenase. Once the cellular supply of ferredoxin has been largely converted to reduced ferredoxin then a) trying to move more electrons to ferredoxin gets harder and b) there is enough reduced ferredoxin to be worth activating metabolism and growth c) this can be initiated by transferring electrons on to stored oxygen (possibly derived from radiolysis or photolysis of water) and allow the superoxide generated to perform the process of converting one metabolite to another.
Ferredoxin (and even ATP, once evolved) could then be used for the more obscure reactions that might require more complicated metabolite interconversions, possibly not amenable to simple ROS mediated methods.
We are still using superoxide today, from reverse electron transport through complex I (directly comparable to that of the prototypical hydrogenase), as the core control of metabolism (pax NOX enzymes). The above speculative narrative would start with superoxide as the actual catalyst at first, one step removed from the origin of life, rather than as a signal. It will be interesting to see whether the modern production of superoxide has any residual enzymic function per se or whether it is now merely a signal/mediator, working through modification of functional sulphydryl groups on proteins which now perform their essential redox catalysis deep within their active sites.
I find it a fascinating idea.
Peter
Addendum. These papers were formative of the above ideas but are a bit like excess baggage to the core principle. I enjoyed them so here they are:
You can do other interesting things with sources of electrons and core members of the modern TCA. If you would like to decarboxylate oxaloacetate to malonate just add ROS:
Malonate as a ROS product is associated with pyruvate carboxylase activity in acute myeloid leukaemia cells
"We have shown that malonate can be formed from oxaloacetate by chemical conversion under the influence of hydrogen peroxide..."
The ROS in this paper which convert oxaloacetate to malonate appears to come from the pyruvate carboxylase enzyme. This is their previous paper which they cited above:
Metabolomic Profiling of Drug Responses in Acute Myeloid Leukaemia Cell Lines
"However, in vitro treatment of oxaloacetate and pyruvate confirm that these conversions are in fact induced by hydrogen peroxide as shown in Figure S4."
"In addition, previous reports have established that ROS mediate the non-enzymatic conversions including that of α-ketoglutarate into succinate [24]-[26]."
This is the alpha-ketoglucarate paper:
Nonezymatic formation of succinate in mitochondria under oxidative stress
"We have shown that malonate can be formed from oxaloacetate by chemical conversion under the influence of hydrogen peroxide..."
The ROS in this paper which convert oxaloacetate to malonate appears to come from the pyruvate carboxylase enzyme. This is their previous paper which they cited above:
"However, in vitro treatment of oxaloacetate and pyruvate confirm that these conversions are in fact induced by hydrogen peroxide as shown in Figure S4."
"In addition, previous reports have established that ROS mediate the non-enzymatic conversions including that of α-ketoglutarate into succinate [24]-[26]."
This is the alpha-ketoglucarate paper:
Nonezymatic formation of succinate in mitochondria under oxidative stress
"The occurrence of nonenzymatic oxidation of KGL in mitochondria was established by an increase in the CO2 and succinate levels in the presence of the oxidants and inhibitors of enzymatic oxidation. H2O2 and menadione as an inductor of reactive oxygen species (ROS) caused the formation of CO2 in the presence of sodium azide and the production of succinate, fumarate, and malate in the presence of rotenone. These substrates were also formed from KGL when mitochondria were incubated with tert-BuOOH at concentrations that completely inhibit KGDH. The nonenzymatic oxidation of KGL can support the TCA cycle under oxidative stress..."
Okay, that will do!