The hypothesis paper
New Control of Mitochondrial Membrane Potential and ROS Formation – A Hypothesis
mentions some factors which might flood mitochondria with Ca2+. Vasopressin gets a mention, again an excellent pressor drug for intractable hypotension, if you don't mind the calcium.
But of course the hormone we all want to ask about is insulin. So you go to Pubmed and search on "insulin calcium ROS"
which brings up this as pretty much the first hit
I haven't read the paper, all I wanted to know was whether insulin behaved as a "stress" hormone as regards Ca2+:
"The insulin-dependent Ca(2+) released from IP3R of skeletal muscle also promotes mitochondrial Ca(2+) uptake."
"The insulin-dependent Ca(2+) released from IP3R of skeletal muscle also promotes mitochondrial Ca(2+) uptake."
It does. Okay, slightly more interesting (and more thoroughly read) is this one:
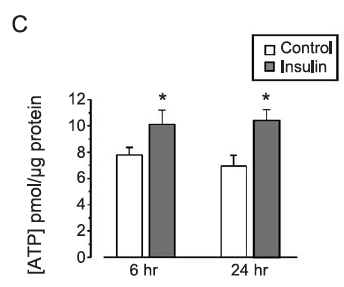
Insulin enhances mitochondrial inner membrane potential and increases ATP levels through phosphoinositide 3-kinase in adult sensory neurons
A lot of cautions yet again. It's neural tissue, which is not a typical insulin sensitive tissue and the paper is old enough that measuring mitochondrial membrane potential was a bit more difficult than ordering a fluorescent dye kit form some generic laboratory supply company.
However it does seem that in something resembling real cells that insulin not only increases delta psi but it also increases ATP levels. Of course we don't know whether the extra ATP comes from glycolysis or increased ox phos from this paper. We do know that it increases.
The insulin concentration used here is 1.0nM which is essentially equivalent to maximal physiological concentration in the aftermath of a meal of modern junk food. Here is the pattern of mitochondrial hyper polarisation at exposure to increasing insulin concentrations. Using 0.75nM is absolutely physiological (if you eat junk food). The glucose used appears to be in the region of 10mM (Ham's F12 medium) and the medium is serum (ie fatty acid) free:
Certain things are clear. There is no dose-response to insulin. Even physiological levels produce a maximal rise in membrane potential. If the pre-insulin membrane polarisation is around 100mV (the technique to assess polarisation couldn't give an absolute value in 2004) then insulin will double this. That seems pretty well certain to generate a membrane polarisation well over the 140mV which will generate copious ROS.
Next thing from here (again)
is that insulin signaling, as assessed by the proportion of Akt which is phosphorylated, is also maximal at exposure to insulin at modestly greater than peak physiological levels (here insulin at 5nM with glucose at ~5mM, some bovine serum and glutamate):
This leaves me with an unanswered question.
We know that we can increase Akt phosphorylation during massively supra-physiological insulin exposure by simply limiting delta psi with agents such as BAM15 or DNP. This is because we limit the ROS generation which is needed to induce insulin-induced insulin resistance, allowing a little extra pAkt to be formed before ROS exceed a critical threshold. We also know that DNP at in-vivo concentrations does the opposite, it reduces insulin signaling. I won't re-cite the same old papers.
What I would like to know is what the oxidation of linoleic acid does to pAkt levels under physiological insulin exposure, compared to palmitic acid. If it is the generation of ROS which limits the rise in pAkt then the lower ROS generation under linoleic acid oxidation should allow more pAkt formation, with enhanced insulin signalling under physiological conditions, which is essential for the development of obesity.
One of my core tenets is that LA limits the normal resistance to insulin signaling mediated by ROS generation. My opinion is the LA causes insulin resistance only once insulin signaling augmentation has produced distended adipocytes which release FFAs in the face of elevated insulin/glucose. In the pre-obese state LA facilitates insulin signaling. Otherwise you wouldn't get fat.
To my knowledge no one has looked at Akt phosphorylation when specific fatty acids are being metabolised under reasonably physiological insulin and glucose concentrations. It would be great to know if LA allowed more pAkt to be formed.
I'd guess that this would be the case.
I have a few more speculations about FFAs, glucose/insulin and ROS which I might leave for another post as this one is getting unwieldy, yet again.
Peter



11 comments:
For those who are visual learners... my notes https://twitter.com/raphaels7/status/1616852482235457538?s=20&t=VHARkhUOPi_26BduKmHBvA
Peter,
Can soleus pushups really reduce postprandial glucose excursion by 50%.
Would love your comments on the paper.
A potent physiological method to magnify and sustain soleus oxidative metabolism improves glucose and lipid regulation
Marc T. Hamilton, Deborah G. Hamilton, and Theodore W. Zderic
https://doi.org/10.1016%2Fj.isci.2022.104869
Hi Gyan,
I have to say that if the study can be replicated I'm impressed. I had to go to the supplementary data to try and work out exactly what they were asking people to do and even then it's not exactly clear, so a crap manuscript. But just lifting your heels of of the ground repeatedly for hours markedly reduces the need for insulin to remove glucose? Fascinating. You have to see the parallel to BAT.
Peter
I wonder if this explains leg jigglers?
One of those mini excercise bikes, pedals only without a seat, seems to work for me. That should be excercising those same muscle groups and the knee and hip rotation helps the old joints. What's better is a nice bike ride in the fresh air.
Hi, Peter,
yes, I also think it's ROS in the negative feedback mechanism. Just one comment, I only see studies where there is more superoxide generated during PUFA metabolism. Do you have any studies with reduced superoxide generation?
I offer an explanation as well, because H2O2 levels are indeed suppressed by linoleic acid, but this is done by excessive glutathione peroxidase activation. All FFAs increase GPx, but linoleic acid increases it the most. This is why LA can increase insulin sensitivity (temporarily), but this causes increased production of superoxide, generation of secondary autooxidation products (aldehydes 9-ONA, HNE, MDA) from LA.
Increased GPx promotes in long term insulin resistance, this is documented in studies.
And I think the conversion of MDA to malonate by ALDH2 is the key, because malonat supress Complex II and this impairs metabolism of all fats.
Jaromir
Hi Jaromir,
There is another post well on its way. And another after that moving to 4-HNE and why it both suppresses and stimulates ROS generation (the later at complex II interestingly). If it all ends up making sense of course. I never have a complete(d) narrative before I start a thread!
Peter
Peter, I did a little digging, apparently H2O2 really does improve insulin sensitivity. This is consistent with overexpression of GPx causing insulin resistance. And the negative feedback seems to be directly on receptor phosphorylation, but that's too complicated a study for me. If I understand it all correctly, then H2O2 could be causing the slowing of dephosphorylation and thus slowing the return from suppressed sensitivity after high insulin to normal insulin sensitivity. What you think?
„The molecular basis for the potentiating effects of H202 on
insulin-stimulated protein tyrosine phosphorylation is currently
unknown. H202 enhances several insulin’s bioeffects
(31-33), but does not affect insulin binding (51). Furthermore,
when added to intact cells (43, 52,53), Hz02 enhances about
2-fold phosphorylation and activation of the insulin receptor
kinase (cf. also Fig. 5). This, however, is in sharp contrast to
the marked potentiation by H202 of insulin’s effects on tyrosine
phosphorylation of the endogenous proteins and suggests
that H202 may have effects distal to kinase activation. For
example, H202 could inhibit a protein tyrosine phosphatase
that rapidly dephosphorylates pp 180 and other proteins in
insulin-treated cells. In favor of such an hypothesis are our
findings that in H202-pretreated cells pp 180 and the other
protein substrates maintain their fully phosphorylated state
even following a 20-min exposure to insulin as opposed to
their transient phosphorylation in cells treated with insulin
alone (Figs. 1 and 2). Alternatively, Hz02 could impede membrane
to cytosol translocation of proteins, leading to their
accumulation in close proximity with the receptor. Indeed,
HzOz, alone or in combination with vanadate, was shown to
stimulate accumulation of insulin-like growth factor I1 receptors
in plasma membranes of rat adipocytes (52). The capability
of the reducing agent DTT tor everse the effects of H2O2
suggests that the action of H202 could be mediated by oxidation
of critical sulfhydryl group(s).“
H202 Potentiates Phosphorylation of Novel Putative Substrates for the Insulin Receptor Kinase in Intact Fao Cells*
https://pubmed.ncbi.nlm.nih.gov/2542323/
Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase
https://www.pnas.org/doi/abs/10.1073/pnas.0308096101
Akt phosphorylates insulin receptor substrate to limit PI3K-mediated PIP3 synthesis
https://elifesciences.org/articles/66942
Oooooh, some interesting stuff there...
Peter
Peter, if you would like to read my view on insulin resistance originated from papers above, I put it on my blog here:
https://mct4health.blogspot.com/2023/02/insulin-resistance-is-caused-by-lack-of.html
Jaromir
Peter, If you would like to read my view on insulin resistance originated from papers above, I put it on my blog:
https://mct4health.blogspot.com/2023/02/insulin-resistance-is-caused-by-lack-of.html
Jaromir
I'd love to see your critique of the Murburn concept explained in this paper by Manoj and Gideon https://acrobat.adobe.com/link/track?uri=urn:aaid:scds:US:546f84fb-fa3c-301d-ba03-320aa378e9a3
They have published extensively on their Murburn concept. This one explains why Murburn is more tenable than the classical proton pump -membrane potential -rotary protein ATP synthesis theory.
Post a Comment