My definition of insulin resistance is an adaptive response to limit insulin-facilitated metabolic substrate ingress in to a cell when an alternative metabolic substrate is being utilised concurrently. With a few caveats.
This is exactly what Carpentier generated when he infused Intralipid/heparin to supply FFAs continuously during an hyperglycaemic clamp test. Look at the control group (open circles):
With glucose clamped at 20mmol/l from 120min onward insulin eventually rises to ~700pmol/l which suppresses FFA availability toward the end of the clamp to around 0.050mmol/l or lower. At this point the subjects are running their metabolism almost completely on the glucose supplied by the infusion and FFAs are, appropriately, sequestered in to adipocytes.
The filled circles are the same people but this time, still with glucose clamped at 20mmol/l, they cannot suppress FFAs using insulin because the FFAs are being supplied exogenously using Intralipid. Free fatty acid release from adipocytes will still drop to near zero, as in the control situation, but plasma FFAs are artificially maintained exactly at fasting levels by the infusion.
The insulin resistance of fasting is real. This essential insulin resistance is not some "problem" to be "cured". It is the suppression of glucose uptake when fatty acid generated ROS are signalling that glucose is not needed, so insulin mediated glucose uptake is also not currently needed. Conveniently, this leaves glucose free for use by the brain.
Intralipid here supplies almost exactly the FFAs needed to imitate fasting (~0.70mmol/l) at a time when blood glucose is clamped at 20mmol/l and insulin is high. Resisting insulin under these circumstances is NOT pathology. It is purely adaptive. Fatty acids at 0.70mmol/l supply almost all of a subject's metabolic needs outside of the brain. Subjects do not need the glucose uptake which insulin and hyperglycaemia are trying to force on them. So they resist it. I would do the same.
You can "cure" this insulin "resistance" by turning off the lipid infusion. Probably in less than half an hour, extrapolating from Shulman's work.
So what goes wrong in metabolic syndrome?
The issue in metabolic syndrome is that you cannot turn off the supply of free fatty acids by pressing the stop button on an infusion pump full of Intralipid.
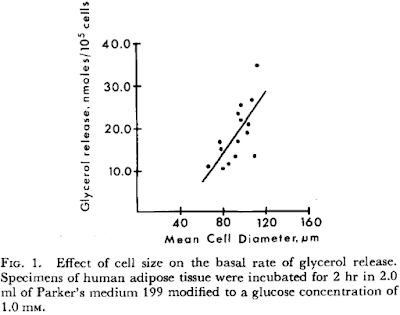
In metabolic syndrome the fatty acids are coming from adipocytes which are larger than they should be and as such have elevated basal lipolysis. We've all read this:
Effect of cell size on lipolysis and antilipolytic action of insulin in human fat cells
Effect of cell size on lipolysis and antilipolytic action of insulin in human fat cells
showing the effect of cell size on basal lipolysis:
and the inability of insulin, even at preposterous dose rates, to suppress this lipolysis:
So the (inappropriate) fatty acid supply to insulin sensitive cells in obesity *requires* insulin resistance. It is derived from large adipocytes, not small adipocytes (which have low rates of basal lipolysis), ie adipose hypertrophy necessitates insulin resistance while adipose hyperplasia does not. At the same fat mass.
Of course it is possible to stop free fatty acid release mediated through basal lipolysis using acipimox. Again, we've all read this one:
and struggled to make out the numbers from its spectacularly low quality pdf file illustrations. I think I have the scales correct here:
The insulin tolerance is markedly improved, clearly, but is it not normal. Also, if you work through the rest of the paper, the mitochondria are still far from normal and I have absolutely no problem with adducts of 4-HNE and its relatives causing problems in their own right within the electron transport chain. They are, after all, an intrinsic part of both insulin's activation and deactivation pathways. I wouldn't ignore them. It's a whole series of potential posts about how and why they might be formed. Or not.
But to get back to metabolic syndrome. The obvious question is "Why are adipocytes so big as to be spilling FFAs through size-related elevated basal lipolysis in the first place?".
Insulin. Insulin makes small fat cells in to large fat cells. Stearate is the most effective fatty acid at generating the ROS signal which limits this, with palmitate a close second. Failure to limit insulin signalling, as neatly demonstrated by safflower oil in the Cocoa study, is what makes an adipocyte excessively insulin sensitive and subsequently engorged. With insulin resistance following on as a secondary change derived from the size of adipocytes.
Linoleic acid is a dud for limiting insulin signalling. It's the pathology.
Peter




No comments:
Post a Comment