Mozaffarian has published this nice observational correlation between three markers of dairy consumption and subsequent onset of diabetes over the following 15 or so years.
Circulating Biomarkers of Dairy Fat and Risk of Incident Diabetes Mellitus Among US Men and Women in Two Large Prospective Cohorts
Any of the three markers is associated with a roughly halving of the incidence of diabetes. I sort of like this, living largely on bulk calories from dairy myself. I also like the size of the effect. If you consider how many people are in the process of developing diabetes in the USA alone, halving that incidence might make a significant contribution to reducing suffering.
If you eat a gram of C15:0 fat, what else comes bundled along with it? Well, in Swedish milk fat there is about a gram of C15:0 in 100g of total fat. That total fat is made up of 70% saturated fats, mostly palmitic acid, a decent dollop of stearic and myristic acids plus odds and sods of shorter chain saturates. These fats might be what reduces the diabetes risk. What makes me think that, other than my biases?
If you feed a normal Bl/6 mouse 40% of its calories as stearic acid, what happens to its blood glucose level? Taken from fig 2.3.1 part B. After 10 weeks the blood glucose of a normal mouse will be significantly lower than if it had been fed standard CIAB or 40% of an oleic acid/PUFA mix. That will be the red arrow:
The blood glucose lowering effect even occurs in db/db mice, a routine model used to vaguely represent T2 diabetes, green arrow. That's all fine and gives some sort of suggestion that it might be the saturated nature of dairy which is protective against diabetes. But why should this occur?
I'm drawn back to the perfused isolated pancreas and the insulin response to physiological levels of glucose in the presence of various fatty acids. This is the image I'm thinking of, from The Insulinotropic Potency of Fatty Acids Is Influenced Profoundly by Their Chain Length and Degree of Saturation:
Notice the marked but transient spike in insulin when glucose is raised from 3.0mmol/l to 12.5mmol/l, most obvious in the black squares representing stearic acid as the background FFA (palmitate is the black triangles). After the spike, which I think represents the first phase insulin response, there is a steady climb in insulin, equivalent to the second phase of insulin secretion. Obviously this is needed because it's an isolate pancreas prep, glucose is fixed at 12.5mmol/l in the perfusate. If the first phase insulin response does its job in real life the systemic circulation (and pancreas) would never see 12.5mmol/l of glucose. The surge of insulin would hit the liver and interact with the insulin receptor. Two things follow on from this. Most insulin would be metabolised following interaction with its receptor, so insulin would never flood the systemic circulation. Second effect is that insulin/insulin receptor activation would shut down hepatic glucose output while the Glut2 transporters continue to pretty well clear the portal vein of glucose.
So a first phase insulin response is designed to protect the systemic circulation from both hyperinsulinaemia and hyperglycaemia. That is its job.
If you want to obliterate the first phase insulin response what you need to do is to reduce reverse electron flow through complex I. Just take a peek at the open circles (oleic acid) and, even better, the closed circles (linoleic acid). For a healthy pancreas, from a healthy rat, you can eliminate the first phase insulin response to hyperglycamia just by choosing your background FFA.
Summary:
Arterycloggingsaturatedfat first phase insulin response 16ng/fraction
vs
Hearthealthypolyunsaturatedfat first phase insulin response 2ng/fraction.
Want to be fat? Use your second phase insulin response in the systemic circulation to pack fat and glucose in to adipocytes. But you must avoid the first phase response because this will keep glucose in the liver as harmless glycogen.
Try using sunflower oil or corn oil as advised by the Food Standards Agency. When you get so fat that your adipocytes start to spew unregulated FFAs, you can be a diabetic (congratulations!). You owe it all to your cardiologist. Or the FSA. Remember who to contact when you get your first diabetic amputation.
Peter
Oh, picked up this quote about dairy and heart disease from Tom Naughton's blog:
"Rather than suggesting that the saturated fats in dairy products are harmless, Aslibekyan and co-author Ana Baylin, an adjunct assistant professor of community health at Brown, hypothesize that other nutrients in dairy products are protective against heart disease, for all but perhaps the highest dairy consumption quintile in their study. The potentially beneficial nutrients include calcium, vitamin D, potassium, magnesium and conjugated linoleic acid (CLA)".
The authors are completely wrong in the interpretation of their own data. On every front. Saturated fats are NOT harmless. Shout it from the roof tops. THEY ARE PROTECTIVE.
"calcium, vitamin D, potassium, magnesium and conjugated linoleic acid (CLA)"?
Bollocks. Ask the mice on stearic acid.
Friday, April 22, 2016
Tuesday, March 29, 2016
Stearate, butter and leptin receptors: Speculation!
I suppose the first thing I have to say is that the Tatter Paper of the last blog entry is not science in the form that any scientist might recognise. Control your variables is rule one... I only posted on it because, by a complete and apparently unplanned accident, the deep fried chips were of similar macros to the boiled mashed potatoes (BMP) but differed in fat type, in a manner very exciting from a Protons point of view. Just to emphasise: This was a chance gem in a pile of wallanga*. Picking the gem out of the wallanga can get your fingers dirty, but it's worth it. Chance is occasionally very useful.
I also happened to notice that, again by chance, the BMP macros were going to pan out somewhere near 40% fat, using butter as the bulk calorie source. This too is quite exciting.
Here are the BMP's macros, just roughed out to round numbers:
The post before the Tatter Paper post was merely pointing out that control C57Bl/6 mice do NOT become obese on their high (40% of calories, chance, neat huh?) fat diet in Ms Reeves' PhD. Their weight might be a little heavier on an olive oil based diet with generous PUFA and a little lighter on a stearic acid based diet with minimal PUFA, but nothing dramatic and absolutely no obesity in sight.
From the 40% fat fed mice in the stearic acid PhD:
Not a perfect match but reasonable. Why is this interesting?
My (repetitive) idea is that a certain amount of input to the ETC via ETFdh drives reverse electron flow through complex I to limit adipocyte distention. Stearic acid plus chow starch does this early during a meal. Butter plus potato starch does this early during a meal. Canola oil plus potato starch doesn't.
We have no idea whether the butter with potato starch would carry on, long term, to a slim phenotype in people. The long term effect of stearate is downwards and of oleate/linoleate is upwards on bodyweight in mice, but the effect is small. How come?
High level signalling.
Anyone who has read Hyperlipid over the last few years will be well aware that I hate high level signalling. It usually takes a basic process, like the control of insulin signalling by the ratio of inputs at complex I, ETFdh and mtG3Pdh, and sticks a nice, glossy, superficial and somewhat opaque surface veneer over it. Then researchers can go off to find 25 or more genes which have some level of influence at some level of "higher-ness" of signalling above the core process. We then end up with a morass of over information with no one linking it all to the core process.
Such a high level signalling molecule is leptin. I have had relatively little interest in leptin over the years so may well be missing large chunks of information which are common knowledge to others on tinternet. The basic process seems to be that fat cells make leptin and the hypothalamus uses the information embedded in blood leptin levels to make a ton of decisions about energy homeostasis and energy use. Including appetite. Leptin secretion is related to adipocyte size but deeply under pinning adipocyte size is the ETC's control of insulin signalling, which sets cellular fat content. Leptin appears to provide some long term modulation of a series of repeated short term post prandial insulin events.
We can strip off the surface veneer of longer term leptin signalling from the core mitochondrial process by using db/db mice. The db/db mouse has non-functional leptin receptors. This means that the acute effect of mitochondrial signalling within adipocytes is not smoothed over or averaged out by the brain using leptin. The core process in fat cells takes over and can be seen via body weight and fat mass.
At peak energy flux stearic acid generates the maximum resistance to insulin's distending effect on adipocytes. Oleic/linoleic is far less able to generate insulin resistance to limit calorie ingress to each adipocyte.
The mice which are db/db homozygous become obese on chow (17% fat largely PUFA). On the 40% oleic/linoleic acid diet they become even more obese because they have plenty of dietary fat to store and a minimal ability to resist insulin's storage signalling. Stearic acid fed db/db mice also have a ton of fat available for storage. They don't lose any weight but they don't gain any weight either and they still up pretty damned close to normal mice fed normal chow. A little heavier, but not much:
The point of this post is emphasise that saturated fat, which I consider to drive physiological adipocyte insulin resistance, limits weight gain in leptin receptor KO mice. The fact it also cures their diabetes at the same time is another story.
Summary so far:
I consider that leptin smooths out the differences in fat storage produced by superoxide signalling originating from the ETC. I hate this, being a great fan of superoxide signalling. Stearate generated superoxide can largely offset the obesogenic effect of being a db/db mouse produced by the attendant lack of leptin signalling. It works with stearate at 40% of the diet, but not at 17% (elsewhere in the PhD).
The rest of this post is wild speculation originating from combining the Stearate PhD and the Tatter Paper:
Could this combo of spuds (or any other starch), butter (38% of calories, quite similar to 40% of calories from stearate) and meatballs work for people who are db/db in the same way as the stearate diet works in db/db mice?
Even more wild speculation, because the db/db genotype is fairly uncommon in humans:
Could the butter component of the "tatters" side-step failed leptin signalling (as in obesity) or relative/absolute hypoleptinaemia (as in the post-obese) in the same way that a ketogenic diet side steps the need for insulin signalling?
Further wild speculation:
Could putting a human on to a high saturated fat ketogenic diet sidestep most of the obesity problems currently prevalent in the world? By giving actual weight loss...
Make up your own mind...
Peter
A final comment on leptin. I'm unfamiliar with the massive complexity of leptin signalling. It seems to go on and on for ever. But just occasionally you come across little snippets of interest which suggest things about the function of leptin. There is a group who have developed an adipocyte specific leptin receptor knockout model. The only cells to lose their leptin receptors are the adipocytes. They still make leptin, they still release leptin, the liver still sees leptin, the hypothalamus still sees leptin. What happens?
"Despite a normal level of leptin receptors in the hypothalamus and normal food intake, mutant mice developed increased adiposity, decreased body temperature, hyperinsulinemia, hypertriglyceridemia, impaired glucose tolerance and insulin sensitivity, as well as elevated hepatic and skeletal muscle triglyceride levels".
The mice become obese and diabetic (on chow of course). Just by their adipocytes failing to perceive plasma leptin levels. And folks think the brain controls obesity! And of course you should be able to fix these adipocytes by supplying stearic acid as 40% of the diet.
Aside: The brain is clearly important in controlling all sorts of physiology. No one would deny this. Much as the computer of a modern car closely controls engine performance (my sister used to drive a Mitsubishi Lancer Evolution which turned out to develop 270bhp on a rolling road. She'd paid for the 315bhp version. Mitsubishi took out the computer, sent it to Japan for upgrade, refitted it and, hey presto, 315bhp. Never touched the engine), so too does the brain fine tune metabolism. But if metabolism is broken peripherally there's not much point looking in the brain. Trying to upgrade the performance of a Morris Minor by reprogramming its computer would be technically slightly difficult. When I used to tune Morris Minor engines computers still ran on punched cards. But the core process in the engines of a Lancer and a Moggy are the same. End aside.
So, did leptin arise to allow fat cells to monitor the fullness of other fat cells so as to maintain a reasonable level of fat stores? Then the brain started listening in? I don't know, but I find the idea interesting. And of course, the basic control of fat storage at that stage would then have been ETC derived superoxide. A little gets insulin signalling going. A lots shuts insulin signalling down. Insulin signalling, of course, is core. Even today.
Final final comment. This post makes me sound like Ray Peat. Something I find very embarrassing, to say the least.
About the asterisk:
*Wallang! Wallanga: You goin' in dat cave man? It's dark in there. We keep cows in there. We keep sheep in there. We keep pigs in there. Take care you don't step in no wallanga.
It needs a guitar, a folk club and a very long shaggy dog ballad to get you to this punch line.
I also happened to notice that, again by chance, the BMP macros were going to pan out somewhere near 40% fat, using butter as the bulk calorie source. This too is quite exciting.
Here are the BMP's macros, just roughed out to round numbers:
The post before the Tatter Paper post was merely pointing out that control C57Bl/6 mice do NOT become obese on their high (40% of calories, chance, neat huh?) fat diet in Ms Reeves' PhD. Their weight might be a little heavier on an olive oil based diet with generous PUFA and a little lighter on a stearic acid based diet with minimal PUFA, but nothing dramatic and absolutely no obesity in sight.
From the 40% fat fed mice in the stearic acid PhD:
Not a perfect match but reasonable. Why is this interesting?
My (repetitive) idea is that a certain amount of input to the ETC via ETFdh drives reverse electron flow through complex I to limit adipocyte distention. Stearic acid plus chow starch does this early during a meal. Butter plus potato starch does this early during a meal. Canola oil plus potato starch doesn't.
We have no idea whether the butter with potato starch would carry on, long term, to a slim phenotype in people. The long term effect of stearate is downwards and of oleate/linoleate is upwards on bodyweight in mice, but the effect is small. How come?
High level signalling.
Anyone who has read Hyperlipid over the last few years will be well aware that I hate high level signalling. It usually takes a basic process, like the control of insulin signalling by the ratio of inputs at complex I, ETFdh and mtG3Pdh, and sticks a nice, glossy, superficial and somewhat opaque surface veneer over it. Then researchers can go off to find 25 or more genes which have some level of influence at some level of "higher-ness" of signalling above the core process. We then end up with a morass of over information with no one linking it all to the core process.
Such a high level signalling molecule is leptin. I have had relatively little interest in leptin over the years so may well be missing large chunks of information which are common knowledge to others on tinternet. The basic process seems to be that fat cells make leptin and the hypothalamus uses the information embedded in blood leptin levels to make a ton of decisions about energy homeostasis and energy use. Including appetite. Leptin secretion is related to adipocyte size but deeply under pinning adipocyte size is the ETC's control of insulin signalling, which sets cellular fat content. Leptin appears to provide some long term modulation of a series of repeated short term post prandial insulin events.
We can strip off the surface veneer of longer term leptin signalling from the core mitochondrial process by using db/db mice. The db/db mouse has non-functional leptin receptors. This means that the acute effect of mitochondrial signalling within adipocytes is not smoothed over or averaged out by the brain using leptin. The core process in fat cells takes over and can be seen via body weight and fat mass.
At peak energy flux stearic acid generates the maximum resistance to insulin's distending effect on adipocytes. Oleic/linoleic is far less able to generate insulin resistance to limit calorie ingress to each adipocyte.
The mice which are db/db homozygous become obese on chow (17% fat largely PUFA). On the 40% oleic/linoleic acid diet they become even more obese because they have plenty of dietary fat to store and a minimal ability to resist insulin's storage signalling. Stearic acid fed db/db mice also have a ton of fat available for storage. They don't lose any weight but they don't gain any weight either and they still up pretty damned close to normal mice fed normal chow. A little heavier, but not much:
The point of this post is emphasise that saturated fat, which I consider to drive physiological adipocyte insulin resistance, limits weight gain in leptin receptor KO mice. The fact it also cures their diabetes at the same time is another story.
Summary so far:
I consider that leptin smooths out the differences in fat storage produced by superoxide signalling originating from the ETC. I hate this, being a great fan of superoxide signalling. Stearate generated superoxide can largely offset the obesogenic effect of being a db/db mouse produced by the attendant lack of leptin signalling. It works with stearate at 40% of the diet, but not at 17% (elsewhere in the PhD).
The rest of this post is wild speculation originating from combining the Stearate PhD and the Tatter Paper:
Could this combo of spuds (or any other starch), butter (38% of calories, quite similar to 40% of calories from stearate) and meatballs work for people who are db/db in the same way as the stearate diet works in db/db mice?
Even more wild speculation, because the db/db genotype is fairly uncommon in humans:
Could the butter component of the "tatters" side-step failed leptin signalling (as in obesity) or relative/absolute hypoleptinaemia (as in the post-obese) in the same way that a ketogenic diet side steps the need for insulin signalling?
Further wild speculation:
Could putting a human on to a high saturated fat ketogenic diet sidestep most of the obesity problems currently prevalent in the world? By giving actual weight loss...
Make up your own mind...
Peter
A final comment on leptin. I'm unfamiliar with the massive complexity of leptin signalling. It seems to go on and on for ever. But just occasionally you come across little snippets of interest which suggest things about the function of leptin. There is a group who have developed an adipocyte specific leptin receptor knockout model. The only cells to lose their leptin receptors are the adipocytes. They still make leptin, they still release leptin, the liver still sees leptin, the hypothalamus still sees leptin. What happens?
"Despite a normal level of leptin receptors in the hypothalamus and normal food intake, mutant mice developed increased adiposity, decreased body temperature, hyperinsulinemia, hypertriglyceridemia, impaired glucose tolerance and insulin sensitivity, as well as elevated hepatic and skeletal muscle triglyceride levels".
The mice become obese and diabetic (on chow of course). Just by their adipocytes failing to perceive plasma leptin levels. And folks think the brain controls obesity! And of course you should be able to fix these adipocytes by supplying stearic acid as 40% of the diet.
Aside: The brain is clearly important in controlling all sorts of physiology. No one would deny this. Much as the computer of a modern car closely controls engine performance (my sister used to drive a Mitsubishi Lancer Evolution which turned out to develop 270bhp on a rolling road. She'd paid for the 315bhp version. Mitsubishi took out the computer, sent it to Japan for upgrade, refitted it and, hey presto, 315bhp. Never touched the engine), so too does the brain fine tune metabolism. But if metabolism is broken peripherally there's not much point looking in the brain. Trying to upgrade the performance of a Morris Minor by reprogramming its computer would be technically slightly difficult. When I used to tune Morris Minor engines computers still ran on punched cards. But the core process in the engines of a Lancer and a Moggy are the same. End aside.
So, did leptin arise to allow fat cells to monitor the fullness of other fat cells so as to maintain a reasonable level of fat stores? Then the brain started listening in? I don't know, but I find the idea interesting. And of course, the basic control of fat storage at that stage would then have been ETC derived superoxide. A little gets insulin signalling going. A lots shuts insulin signalling down. Insulin signalling, of course, is core. Even today.
Final final comment. This post makes me sound like Ray Peat. Something I find very embarrassing, to say the least.
About the asterisk:
*Wallang! Wallanga: You goin' in dat cave man? It's dark in there. We keep cows in there. We keep sheep in there. We keep pigs in there. Take care you don't step in no wallanga.
It needs a guitar, a folk club and a very long shaggy dog ballad to get you to this punch line.
Monday, March 21, 2016
Boiled mashed potatoes for miracle satiety?
The effects of potatoes and other carbohydrate side dishes consumed with meat on food intake, glycemia and satiety response in children.
With thanks to Mike Eades for the full text.
This is an interesting study. Given a meal of meatballs plus a choice of five different carbohydrate sources, a group of children ate a great deal less (in calories) of boiled mashed potatoes than of pasta, rice or either of two types of chips.
"The five treatment sessions consisted of ad libitum servings of (i) rice, (ii) pasta, (iii) boiled and mashed potato (BMP), (iv) baked French fries (BFF) and (v) fried French fries (FFF) with a fixed amount (100 g) of meatballs".
What did they find?
"... children consumed 30–40% less calories at meals with BMP (p less than 0.0001) compared with all other treatments, which were similar".
That's a LOT less calories! Potatoes seem to have some sort of magical satiety property. If you believe in magic. Table 1 gives an inkling of the problems with the study:
As you read through the cooking description you realise (red box) that the carbohydrates had very different amounts of added fat per unit carbohydrate and that some had butter (+/- added milk) while others had canola oil in varying doses. So when we look at Table 3 we have to realise that "CHO amount (g)" means an assorted mix of various fats and carbs:
We have to work back using Table 1 to find out what amounts of carbohydrate and fat were actually eaten and read the cooking details to find out what the fats were in each dish. Some arithmetic gives us this for what was actually eaten:
To my mind the trial here splits in to two. We have BMP, boiled mashed potatoes with 3g of carbohydrate per gram of butter, which is fairly well matched with FFF, chips deep fried in canola oil, with 2g of carbohydrate per gram of canola oil. Both are potatoes. Both provide a roughly similar ratio of calories/grams from glucose and fat. Both are relatively low carbohydrate per unit fat (compared to the other three meals, ie just in this study).
From the Protons point of view the relatively low carb BMP and FFF are supplying glucose from potatoes to drive complex I. However butter also supplies FADH2 at ETFdh, so generates a resistance within adipocytes (and elsewhere) to an excessive insulin facilitated calorie ingress during the period of maximal blood nutrient levels. When calories stop falling in to adipocytes, satiety kicks in. Using FADH2 this happens after eating 508 kcal. With FFF based on canola oil, ie potatoes steeped in 18 carbon omega 3 and 6 PUFA, the beta oxidation generates a much lower input at ETFdh (one less FADH2 per double bond) and so insulin sensitivity at peak nutrient uptake is maintained for longer, fat pours in to adipocytes for longer and almost twice as many calories are consumed (912 kcal) before satiety kicks in. I expect satiety to rise as blood nutrients rise. Not sequestering them in to adipocytes seems the best way to do this. More physiological insulin resistance. I'm guessing the brain does the actual sensing of both glucose and FFAs.
I like that. You can say what you like about the hypothalamus. I prefer to think about the adipocytes and their mitochondria as determining what gets done with food and hunger. There is some input from leptin of course, but that's another post.
The other three carbohydrate dishes are essentially lowish fat foods with between 7g and 10g of carbohydrate per gram of butter or canola oil.
In these lower fat preparations it takes three or four teaspoons of butter to generate satiety vs just under 6 teaspoons of canola oil, roughly twice as much fat is needed when carried with a similar amount of starch. A reasonable fit with a Protons point of view, though not as pleasing as the BMP vs FFF comparison.
How the study was developed is fascinating to think about.
What decisions were made at the planning stage? Obviously, someone had worked out, well before any grant application was submitted, that higher saturated fat with lower carb meals are by far the most satiating. Or maybe they are dumb and they were just lucky to get a result? Personally, I can't see how you engineer a study like this unless you are pretty clever and well informed, not at the mitochondrial level of course, but certainly at the butter level. Mashed potatoes, which already have something of a reputation as a miracle weight loss food, getting a helping hand... From a dollop of butter. It makes sense.
BTW this is Canada. I can't see how such a study would ever have gotten past any ethics review committee in the US of A. Imagine trying to feed BUTTER to American children. Immoral. Plus they might not eat up their carbs!
Peter
With thanks to Mike Eades for the full text.
This is an interesting study. Given a meal of meatballs plus a choice of five different carbohydrate sources, a group of children ate a great deal less (in calories) of boiled mashed potatoes than of pasta, rice or either of two types of chips.
"The five treatment sessions consisted of ad libitum servings of (i) rice, (ii) pasta, (iii) boiled and mashed potato (BMP), (iv) baked French fries (BFF) and (v) fried French fries (FFF) with a fixed amount (100 g) of meatballs".
What did they find?
"... children consumed 30–40% less calories at meals with BMP (p less than 0.0001) compared with all other treatments, which were similar".
That's a LOT less calories! Potatoes seem to have some sort of magical satiety property. If you believe in magic. Table 1 gives an inkling of the problems with the study:
As you read through the cooking description you realise (red box) that the carbohydrates had very different amounts of added fat per unit carbohydrate and that some had butter (+/- added milk) while others had canola oil in varying doses. So when we look at Table 3 we have to realise that "CHO amount (g)" means an assorted mix of various fats and carbs:
We have to work back using Table 1 to find out what amounts of carbohydrate and fat were actually eaten and read the cooking details to find out what the fats were in each dish. Some arithmetic gives us this for what was actually eaten:
To my mind the trial here splits in to two. We have BMP, boiled mashed potatoes with 3g of carbohydrate per gram of butter, which is fairly well matched with FFF, chips deep fried in canola oil, with 2g of carbohydrate per gram of canola oil. Both are potatoes. Both provide a roughly similar ratio of calories/grams from glucose and fat. Both are relatively low carbohydrate per unit fat (compared to the other three meals, ie just in this study).
From the Protons point of view the relatively low carb BMP and FFF are supplying glucose from potatoes to drive complex I. However butter also supplies FADH2 at ETFdh, so generates a resistance within adipocytes (and elsewhere) to an excessive insulin facilitated calorie ingress during the period of maximal blood nutrient levels. When calories stop falling in to adipocytes, satiety kicks in. Using FADH2 this happens after eating 508 kcal. With FFF based on canola oil, ie potatoes steeped in 18 carbon omega 3 and 6 PUFA, the beta oxidation generates a much lower input at ETFdh (one less FADH2 per double bond) and so insulin sensitivity at peak nutrient uptake is maintained for longer, fat pours in to adipocytes for longer and almost twice as many calories are consumed (912 kcal) before satiety kicks in. I expect satiety to rise as blood nutrients rise. Not sequestering them in to adipocytes seems the best way to do this. More physiological insulin resistance. I'm guessing the brain does the actual sensing of both glucose and FFAs.
I like that. You can say what you like about the hypothalamus. I prefer to think about the adipocytes and their mitochondria as determining what gets done with food and hunger. There is some input from leptin of course, but that's another post.
The other three carbohydrate dishes are essentially lowish fat foods with between 7g and 10g of carbohydrate per gram of butter or canola oil.
In these lower fat preparations it takes three or four teaspoons of butter to generate satiety vs just under 6 teaspoons of canola oil, roughly twice as much fat is needed when carried with a similar amount of starch. A reasonable fit with a Protons point of view, though not as pleasing as the BMP vs FFF comparison.
How the study was developed is fascinating to think about.
What decisions were made at the planning stage? Obviously, someone had worked out, well before any grant application was submitted, that higher saturated fat with lower carb meals are by far the most satiating. Or maybe they are dumb and they were just lucky to get a result? Personally, I can't see how you engineer a study like this unless you are pretty clever and well informed, not at the mitochondrial level of course, but certainly at the butter level. Mashed potatoes, which already have something of a reputation as a miracle weight loss food, getting a helping hand... From a dollop of butter. It makes sense.
BTW this is Canada. I can't see how such a study would ever have gotten past any ethics review committee in the US of A. Imagine trying to feed BUTTER to American children. Immoral. Plus they might not eat up their carbs!
Peter
Monday, February 15, 2016
High fat fed mice on stearic acid
The concept of finding anything positive about palmitic acid is still tantamount to research suicide. However, stearic acid is a rather different matter. It's lipid "neutral" for those poor folks who still bow their heads and kneel before the altar of the lipid hypothesis. So you can publish good stuff about stearic acid with relative impunity.
Raymond sent me the PhD thesis of Valerie Reeves, Kentucky University.
Before we think about leptin receptor defective mice (another day), we can ask questions about the control groups. Such as:
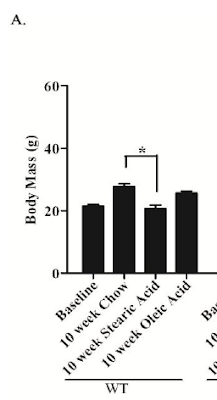
What happens if you feed a fairly typical C57Bl/6 mouse 40% of its calories from fat, based on fully saturated stearic acid?
They stay significantly slimmer than they do on CIAB (chow) and probably slimmer than when fed on 40% oleic acid (olive oil w/o the PUFA).
(EDIT As Tucker pointed out in comments: You might be able to explain the relative weight gains in terms of omega 6 PUFA. Chow was about 13% of energy as PUFA, stearic acid diet about 5% PUFA and the oleic acid diet about 14% PUFA. The correlation of PUFA with fat gain isn’t perfect but it’s quite close… END EDIT)
Now this is clearly impossible, as anyone who has read anything about Bl/6 mice and fat will be very aware. So the poor girl did it again:
This time we have p values sprouting all over the graph like mould in a Winter bathroom. For mice, chow makes you fat. Olive oil makes you fat. Stearic acid doesn't. Impossible I know, but that's twice it has happened. For fat mass the p values never make pay dirt but the writing is on the wall for oleic acid and fat gain too:
The wild type control mice were so nice in this PhD thesis that I thought I'd just put up these few figures before we consider what might happen if (gasp) you put an obese, diabetic db-/- mouse on a highly saturated stearic acid based diet.
I think palmitic acid would do exactly the same as stearic acid did for these mice. But who would risk their career with a finding like that? The corollary is that when you see a C57Bl/6 mouse get fat on a high fat diet, you know there are lots of double bonds in that fat........
Peter
Raymond sent me the PhD thesis of Valerie Reeves, Kentucky University.
Before we think about leptin receptor defective mice (another day), we can ask questions about the control groups. Such as:
What happens if you feed a fairly typical C57Bl/6 mouse 40% of its calories from fat, based on fully saturated stearic acid?
They stay significantly slimmer than they do on CIAB (chow) and probably slimmer than when fed on 40% oleic acid (olive oil w/o the PUFA).
(EDIT As Tucker pointed out in comments: You might be able to explain the relative weight gains in terms of omega 6 PUFA. Chow was about 13% of energy as PUFA, stearic acid diet about 5% PUFA and the oleic acid diet about 14% PUFA. The correlation of PUFA with fat gain isn’t perfect but it’s quite close… END EDIT)
Now this is clearly impossible, as anyone who has read anything about Bl/6 mice and fat will be very aware. So the poor girl did it again:
This time we have p values sprouting all over the graph like mould in a Winter bathroom. For mice, chow makes you fat. Olive oil makes you fat. Stearic acid doesn't. Impossible I know, but that's twice it has happened. For fat mass the p values never make pay dirt but the writing is on the wall for oleic acid and fat gain too:
The wild type control mice were so nice in this PhD thesis that I thought I'd just put up these few figures before we consider what might happen if (gasp) you put an obese, diabetic db-/- mouse on a highly saturated stearic acid based diet.
I think palmitic acid would do exactly the same as stearic acid did for these mice. But who would risk their career with a finding like that? The corollary is that when you see a C57Bl/6 mouse get fat on a high fat diet, you know there are lots of double bonds in that fat........
Peter
Not really much about swimming underwater (2)
Just a one liner after all the discussions about breath holding on a fat based diet:
Effects of Twenty Days of the Ketogenic Diet on Metabolic and Respiratory Parameters in Healthy Subjects.
The first person I came across doing this practically was a LC blogger back in my early days (probably 2002-ish) and I didn't realise why she was LC eating to manage her chronic lung disease, from the metabolic perspective. She was very focused on saturated fat, obviously (with hind sight!). I've not been through the above link's full text but you all know the depths of stupidity of most saturophobes. If this was corn oil and MCT based... Perhaps we could get a significant O2 consumption drop given some butter, dunno. Nice to see some medics taking this seriously!
Peter
With apologies to whoever put this up on Facebook, one of those saved links but no idea who it came from.
Effects of Twenty Days of the Ketogenic Diet on Metabolic and Respiratory Parameters in Healthy Subjects.
The first person I came across doing this practically was a LC blogger back in my early days (probably 2002-ish) and I didn't realise why she was LC eating to manage her chronic lung disease, from the metabolic perspective. She was very focused on saturated fat, obviously (with hind sight!). I've not been through the above link's full text but you all know the depths of stupidity of most saturophobes. If this was corn oil and MCT based... Perhaps we could get a significant O2 consumption drop given some butter, dunno. Nice to see some medics taking this seriously!
Peter
With apologies to whoever put this up on Facebook, one of those saved links but no idea who it came from.
Tuesday, February 09, 2016
Life (11) Ferredoxin
Anyone who has read through the Life series will know that I have a great deal of time for reduced FeS moieties as the core energy source used during the transition between pre-biotic chemistry and something resembling life.
Bottom line: An electron from a reduced FeS moiety is able to reduce dissolved CO2 to a Ni bound CO- group using molecular hydrogen. If you supply a couple of geochemical CH3-SH molecules you can then generate acetyl-SH, precursor to acetyl-CoA, from this Ni-CO. After that it's down hill all the way to metabolism. This is where the watchmaker comes from who is going to make the watch which you might find in the jungle.
One of the earliest biological problems was to detach this reduced FeS from the inorganic cell wall and make it mobile. The solution is ferredoxin.
People have looked at the ferredoxin used by those bacteria which have done well for several billion years by developing the form of metabolism most closely allied to this very basic pre biotic chemistry. One of these ferredoxins was sequenced very early in the 1960's, while I was just a kid playing in the streets of Nottingham and Mike Russell (thanks Jack) had just gained his geology BSc from London University.
Working with the ferredoxin sequence from Clostridium pasteurianum Eck and Dayhoff noticed some interesting things. There looks to have been a very early gene duplication, this allows the two sections of the protein to be compared to each other and this facilitated all sorts of speculation about its possible origin. A sort of molecular Rosetta Stone. Here is the sequence they started from in the nicely descriptive three letter code (it becomes more legible if you click on it):
To make comparisons easier to fit on a given line they then changed the three letter notation to the less descriptive single letter notation for amino acids during the rest of the discussion, which goes like this:
The legend to Fig 1 is quite self explanatory but, if anyone wants the full text to work though it line by line in more detail, I have the pdf. The end conclusion is that primordial ferredoxin could be derived from a simple repeating pattern of just 4 amino acids. These four:
From these four amino acids they suggest you can reverse engineer the process giving this as the process of generating ferredoxin:
Apart from a nice discussion about why a very early protein, given billions of years to evolve, remains so remarkably similar to its primordial sequence, they also have a think about what the ADSG polymer might have been doing before it was co-opted to pick up an FeS cluster. Possibly some sort of simple structural polymer. They also throw in the concept that the FeS might initially have been only chelated to cysteine, I would suggest as a solubilising agent. Again, cysteine is one of the most primordial of amino acids:
I found the paper and its proposals fascinating. They are talking about concepts which fit extremely well in to Mike Russell's ideas about hydrothermal vents at a time before there was any evidence that the vents existed. As far as I can tell it has no bearing on anything we might do today but I still like it. It says a great deal about where we might have come from.
Peter
Bottom line: An electron from a reduced FeS moiety is able to reduce dissolved CO2 to a Ni bound CO- group using molecular hydrogen. If you supply a couple of geochemical CH3-SH molecules you can then generate acetyl-SH, precursor to acetyl-CoA, from this Ni-CO. After that it's down hill all the way to metabolism. This is where the watchmaker comes from who is going to make the watch which you might find in the jungle.
One of the earliest biological problems was to detach this reduced FeS from the inorganic cell wall and make it mobile. The solution is ferredoxin.
People have looked at the ferredoxin used by those bacteria which have done well for several billion years by developing the form of metabolism most closely allied to this very basic pre biotic chemistry. One of these ferredoxins was sequenced very early in the 1960's, while I was just a kid playing in the streets of Nottingham and Mike Russell (thanks Jack) had just gained his geology BSc from London University.
Working with the ferredoxin sequence from Clostridium pasteurianum Eck and Dayhoff noticed some interesting things. There looks to have been a very early gene duplication, this allows the two sections of the protein to be compared to each other and this facilitated all sorts of speculation about its possible origin. A sort of molecular Rosetta Stone. Here is the sequence they started from in the nicely descriptive three letter code (it becomes more legible if you click on it):
To make comparisons easier to fit on a given line they then changed the three letter notation to the less descriptive single letter notation for amino acids during the rest of the discussion, which goes like this:
The legend to Fig 1 is quite self explanatory but, if anyone wants the full text to work though it line by line in more detail, I have the pdf. The end conclusion is that primordial ferredoxin could be derived from a simple repeating pattern of just 4 amino acids. These four:
From these four amino acids they suggest you can reverse engineer the process giving this as the process of generating ferredoxin:
Apart from a nice discussion about why a very early protein, given billions of years to evolve, remains so remarkably similar to its primordial sequence, they also have a think about what the ADSG polymer might have been doing before it was co-opted to pick up an FeS cluster. Possibly some sort of simple structural polymer. They also throw in the concept that the FeS might initially have been only chelated to cysteine, I would suggest as a solubilising agent. Again, cysteine is one of the most primordial of amino acids:
I found the paper and its proposals fascinating. They are talking about concepts which fit extremely well in to Mike Russell's ideas about hydrothermal vents at a time before there was any evidence that the vents existed. As far as I can tell it has no bearing on anything we might do today but I still like it. It says a great deal about where we might have come from.
Peter
Tuesday, February 02, 2016
Insulin glucagon and protein
Again from dissertante's query: How can chicken be found to raise blood glucose, acutely?
Many years ago, as a beginner at treating diabetic animals, I tried to balance insulin dose rate/timing against carbohydrate intake. Owners always asked if there was anything they could feed as treats etc. I used to suggest meat and fat as they shouldn't need insulin for processing.
This was a mistake. Dogs are, by the time we diagnose them, functionally type 1 diabetics. While fat is perfectly OK, protein certainly isn't.
Eating protein, for a type 1 diabetic, produces an immediate rise in blood glucose. This is nothing to do with gluconeogenic amino acids, the effect of which would expect to be delayed for several hours, if it occurs at all. While protein for an normal human being/animal is neutral on systemic blood glucose it never the less produces an immediate spike (by around 60 minutes) in blood insulin.
Dandona measured insulin and glucose, although not glucagon, after casein ingestion as we saw in the last post:
Eating 75g of casein protein more or less triples your blood insulin level but doesn't budge blood glucose down any more than cream does, which leaves insulin pretty well alone. Under normal conditions the casein induced spike in insulin is counterbalanced by a rise in glucagon. If the insulin rise does not occur (through beta cell failure) the glucagon will still rise and is unopposed, so hyperglycaemia is the net result, coming from a rise in hepatic glucose output.
This took me years to realise. Slow, I know but ah well.... It's now common knowledge and Dr Unger's glucagonocentric view of diabetic hyperglycaemia makes a great deal of sense.
So protein will provoke hyperglycaemia in the absence of an insulin response, via glucagon, in a type 1 diabetic. I would guess that the same would apply to an advanced type 2. It very recently occurred to me that an elevated blood glucose after protein intake might be a useful supplementary test for certain oddities in OGTTs.
I had an email a few weeks ago about OGTT results in long term, non diabetic low carb eaters. I don't know the exact details of duration of LC eating or the period of carb loading before the OGTT, but the end result after glucose ingestion was a sustained hyperglycaemia with profoundly depressed C-peptide levels.
The worry here is that long term LC might have led to endocrine pancreatic insufficiency. My initial thought was to wonder what the response to exogenous insulin might be, but this was probably the wrong line of thought.
What would be far more interesting would be to run an oral protein response test, looking at blood glucose, insulin, glucagon and C-peptide. Although, at a pinch, all you need is the blood glucose result. If a person has developed a significant loss of beta cells then the unopposed alpha cell glucagon response to this protein would produce hyperglycaemia. A normal insulin reaction in response to protein would produce normoglycaemia after said protein load.
We all know that after a month or two of LC eating that three days at 150g/d of carbs will restore a normal response to glucose. But the question is what time scale of carb loading is needed after several years of LC eating. The regulation of insulin secretion in response to glucose requires active glycolysis, regulated by glucokinase in the pancreas. Glucokinase gene expression is controlled by dietary glucose supply. If long term glucokinase down regulation takes longer than a few days of carbohydrate loading to reverse, this would produce intolerance to glucose but would have no effect on insulin secretion when driven by amino acids. It would be quite simple to differentiate between down regulation of the pancreatic glucose sensor from newly acquired type 1 diabetes during LC eating.
Summary: Elevated blood glucose after an oral protein load suggests genuine diabetes. Poor responsiveness to glucose after sustained LC eating simply reflects a mothballed glucose sensor, provided response to protein is normal.
Peter
Many years ago, as a beginner at treating diabetic animals, I tried to balance insulin dose rate/timing against carbohydrate intake. Owners always asked if there was anything they could feed as treats etc. I used to suggest meat and fat as they shouldn't need insulin for processing.
This was a mistake. Dogs are, by the time we diagnose them, functionally type 1 diabetics. While fat is perfectly OK, protein certainly isn't.
Eating protein, for a type 1 diabetic, produces an immediate rise in blood glucose. This is nothing to do with gluconeogenic amino acids, the effect of which would expect to be delayed for several hours, if it occurs at all. While protein for an normal human being/animal is neutral on systemic blood glucose it never the less produces an immediate spike (by around 60 minutes) in blood insulin.
Dandona measured insulin and glucose, although not glucagon, after casein ingestion as we saw in the last post:
Eating 75g of casein protein more or less triples your blood insulin level but doesn't budge blood glucose down any more than cream does, which leaves insulin pretty well alone. Under normal conditions the casein induced spike in insulin is counterbalanced by a rise in glucagon. If the insulin rise does not occur (through beta cell failure) the glucagon will still rise and is unopposed, so hyperglycaemia is the net result, coming from a rise in hepatic glucose output.
This took me years to realise. Slow, I know but ah well.... It's now common knowledge and Dr Unger's glucagonocentric view of diabetic hyperglycaemia makes a great deal of sense.
So protein will provoke hyperglycaemia in the absence of an insulin response, via glucagon, in a type 1 diabetic. I would guess that the same would apply to an advanced type 2. It very recently occurred to me that an elevated blood glucose after protein intake might be a useful supplementary test for certain oddities in OGTTs.
I had an email a few weeks ago about OGTT results in long term, non diabetic low carb eaters. I don't know the exact details of duration of LC eating or the period of carb loading before the OGTT, but the end result after glucose ingestion was a sustained hyperglycaemia with profoundly depressed C-peptide levels.
The worry here is that long term LC might have led to endocrine pancreatic insufficiency. My initial thought was to wonder what the response to exogenous insulin might be, but this was probably the wrong line of thought.
What would be far more interesting would be to run an oral protein response test, looking at blood glucose, insulin, glucagon and C-peptide. Although, at a pinch, all you need is the blood glucose result. If a person has developed a significant loss of beta cells then the unopposed alpha cell glucagon response to this protein would produce hyperglycaemia. A normal insulin reaction in response to protein would produce normoglycaemia after said protein load.
We all know that after a month or two of LC eating that three days at 150g/d of carbs will restore a normal response to glucose. But the question is what time scale of carb loading is needed after several years of LC eating. The regulation of insulin secretion in response to glucose requires active glycolysis, regulated by glucokinase in the pancreas. Glucokinase gene expression is controlled by dietary glucose supply. If long term glucokinase down regulation takes longer than a few days of carbohydrate loading to reverse, this would produce intolerance to glucose but would have no effect on insulin secretion when driven by amino acids. It would be quite simple to differentiate between down regulation of the pancreatic glucose sensor from newly acquired type 1 diabetes during LC eating.
Summary: Elevated blood glucose after an oral protein load suggests genuine diabetes. Poor responsiveness to glucose after sustained LC eating simply reflects a mothballed glucose sensor, provided response to protein is normal.
Peter
Personalised nutrition: Eat fat
Personalized Nutrition by Prediction of Glycemic Responses
In the comments after the last post, dissertante asked about the above study. It's been around for a while and many folks have talked about it, Bill Lagakos being one of the more articulate. The study is enormous. The paper is quite long and, for various reasons, not exactly gripping reading for myself. So I may well have missed certain facts which are not immediately obvious. This is the summary of the study from the abstract:
My initial thought was to ask how the insulin response varied between people with a normoglycaemic response to junk food vs hyperglycaemic response. Typical junk foods considered in the study are the bananas vs the cookies in section G of Figure 2:
If normoglycaemia is bought at the cost of hyperinsulinaemia, it's not particularly attractive, to me anyway. Banana, cookie, who cares? The only way I can see that either of these is acceptable as food is if they are taken by the gut bacteria, converted to short chain fatty acids and so bypass the whole insulin/glucose signalling system. Many people seem to be happy to trust their health and glycaemic control to their gut bacteria. It takes all sorts I guess.
So, the implication is that we can use this massive level of investigation to make choices between carbs which spike glucose and carbs which don't. For us, on an individual basis, tailored nutrition. Without any idea of what these given sources of carbohydrate do to an individual's insulin levels. But, to be quite honest, it's junk vs junk anyway.
There is a snippet which shows a glimmer of interest in the use of fat to blunt the glycaemic response to carbohydrate by the group. This is what they say:
"The PDP [partial dependence plots, part of their model] of fat exhibits a beneficial effect for fat since our algorithm predicts, on average, lower PPGR [post prandial glucose response] as the meal’s ratio of fat to carbohydrates (Figure 4C) or total fat content (Figure S5A) increases, consistent with studies showing that adding fat to meals may reduce the PPGR (Cunningham and Read, 1989). However, here too, we found that the effect of fat varies across people".
Fat cannot reliably save us from carbohydrate induced hyperglycaemia. We still need personalised nutrition, even if we eat fat.
But what if we eat only fat? What would be the glycaemic response to 100ml of double cream, drunk on its own, for breakfast?
Dandona, on his way to drawing incorrect conclusions, gives us the glucose and insulin data for 100ml of double cream:
Drinking cream alone mildly reduces insulin after a transient rise and point blank drops glucose throughout the study period. There may be minor individual variations in response but these are all contained within standard deviations which narrow with time after exposure... There is little scope for a pathological rise in glucose or insulin within those SDs.
So how much do we have to go begging, cap-in-hand, to our gut microbiota for a nice glucose AND insulin response to 100ml of cream? Not a lot. Ditto butter, lard, beef dripping...
The simple approach to personalised nutrition is to eat fat, cut out the middle man of our microbiota, limit glucose and reduce signalling through the insulin pathway while eating just enough protein to meet our needs. Anything else is going to need an awful lot of laboratory investigations to even get half the information we need to keep our blood glucose levels remotely normal while still using unknown amounts of insulin.
Personalised nutrition: Eat fat.
Peter
Oh, dissertante also mention that, for some people, chicken came through as a "bad" food in terms of post prandial glycaemia. That's another post I guess.
In the comments after the last post, dissertante asked about the above study. It's been around for a while and many folks have talked about it, Bill Lagakos being one of the more articulate. The study is enormous. The paper is quite long and, for various reasons, not exactly gripping reading for myself. So I may well have missed certain facts which are not immediately obvious. This is the summary of the study from the abstract:
My initial thought was to ask how the insulin response varied between people with a normoglycaemic response to junk food vs hyperglycaemic response. Typical junk foods considered in the study are the bananas vs the cookies in section G of Figure 2:
If normoglycaemia is bought at the cost of hyperinsulinaemia, it's not particularly attractive, to me anyway. Banana, cookie, who cares? The only way I can see that either of these is acceptable as food is if they are taken by the gut bacteria, converted to short chain fatty acids and so bypass the whole insulin/glucose signalling system. Many people seem to be happy to trust their health and glycaemic control to their gut bacteria. It takes all sorts I guess.
So, the implication is that we can use this massive level of investigation to make choices between carbs which spike glucose and carbs which don't. For us, on an individual basis, tailored nutrition. Without any idea of what these given sources of carbohydrate do to an individual's insulin levels. But, to be quite honest, it's junk vs junk anyway.
There is a snippet which shows a glimmer of interest in the use of fat to blunt the glycaemic response to carbohydrate by the group. This is what they say:
"The PDP [partial dependence plots, part of their model] of fat exhibits a beneficial effect for fat since our algorithm predicts, on average, lower PPGR [post prandial glucose response] as the meal’s ratio of fat to carbohydrates (Figure 4C) or total fat content (Figure S5A) increases, consistent with studies showing that adding fat to meals may reduce the PPGR (Cunningham and Read, 1989). However, here too, we found that the effect of fat varies across people".
Fat cannot reliably save us from carbohydrate induced hyperglycaemia. We still need personalised nutrition, even if we eat fat.
But what if we eat only fat? What would be the glycaemic response to 100ml of double cream, drunk on its own, for breakfast?
Dandona, on his way to drawing incorrect conclusions, gives us the glucose and insulin data for 100ml of double cream:
Drinking cream alone mildly reduces insulin after a transient rise and point blank drops glucose throughout the study period. There may be minor individual variations in response but these are all contained within standard deviations which narrow with time after exposure... There is little scope for a pathological rise in glucose or insulin within those SDs.
So how much do we have to go begging, cap-in-hand, to our gut microbiota for a nice glucose AND insulin response to 100ml of cream? Not a lot. Ditto butter, lard, beef dripping...
The simple approach to personalised nutrition is to eat fat, cut out the middle man of our microbiota, limit glucose and reduce signalling through the insulin pathway while eating just enough protein to meet our needs. Anything else is going to need an awful lot of laboratory investigations to even get half the information we need to keep our blood glucose levels remotely normal while still using unknown amounts of insulin.
Personalised nutrition: Eat fat.
Peter
Oh, dissertante also mention that, for some people, chicken came through as a "bad" food in terms of post prandial glycaemia. That's another post I guess.
Saturday, January 16, 2016
On drinking varnish
Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet.
Raphi sent me this link early in the New Year. It’s nice. It demonstrates, at some level of complexity, that omega 6 PUFA at 8% of calories are obesogenic in mice, even if they are fed otherwise fat free CIAB. It’s all about endocannabinoid ligands and receptor activation. Potentially useful when folks get round to starting class actions against the cardiological community and any other health advisors warning against saturated fat. If you limit fat to 30% of calories and saturated fat to 10% you still have 20% PUFA/MUFA in your diet. That’s easily obesogenic. Your cardiologist made you fat. Sue now.
But all of this endocannabinoid stuff is what I call high level signalling. At the core mitochondrial level we know that omega 6 PUFA fail to limit insulin activity under situations where a saturated fat would shut down insulin mediated calorie ingress. In an adipocyte this means that, during oxidation of omega 6 PUFA, insulin continues to signal and fatty acids (and glucose) fall in to the adipocytes, stay there, and you get really hungry. Modified chemicals derived from this system of omega six fatty acids are overlaid on top of the core mitochondrial signalling. A modified derivative of arachidonic acid becomes an endocannabinoid ligand and makes you hungry and fat. The system takes something basic and develops an overlay of enormous complexity, this is what I call higher level signalling.
I hate higher level signalling. Give me the core process anyday.
On this front people may realise I have issues with omega 3 PUFA fats. From the ETC perspective they are worse than omega 6 PUFA and should be more obesogenic. But, in general they’re not. In fact there is a massive industry showing us how good they are for us. But there are suggestions that the core process which makes omega 6 PUFA obesogenic really do apply to the omega 3s. Bear in mind that we are only talking about linoleic and alpha linolenic acids here. Longer fatty acids go to peroxisomes for oxidation and have little influence on core mitochondrial processes, though they do perform a great deal of high level signalling. Here we go:
Sucrose counteracts the anti-inflammatory effect of fish oil in adipose tissue and increases obesity development in mice.
Notice the obesogenic effect of fish oil only shows when sucrose is present in the diet. Replacing sucrose with protein eliminates the effect. Fructose is an unstoppable source of cellular energy intake which needs insulin resistance to limit insulin signalling facilitated ingress of glucose. As insulin continues to act, fat cells sequester calories. Fish oil combined with sucrose is the worst, corn oil is intermediate and, without sucrose, none of the fats are obesogenic.
This makes me happy. I can see the core process at work, never mind what EPA and DHA say to g-protein coupled receptors.
There is another paper which shows a similar effect and I like it rather a lot because the cognitive dissonance, which shines through every word of the text, is rather entertaining. How can you get a life-sustaining source of funding if your data show that omega 3 PUFA are grossly obesogenic? They improve insulin signalling exactly as the ETC effects would predict. The cost of improved insulin responsiveness in adipocytes is obesity. Here we go again:
Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids.
The values to look at begin with the weight gain. All we have to do is to subtract weight at the start of the study period from weight at the end (perhaps the authors don't do arithmetic?). Low fat group gained a gram, added saturated fat group gained 0.6 g, added omega 6 group lost* 2.4g and omega 3 group gained 10.4g.
Ten point four grams.
These are db/db mice which lack a functional leptin receptor. They are diabetic and I feel their chronic hyperglycaemia represents a similar drive to obesity as the fructose loading in the last study, ie an unregulated source of calories which drop in to adipocytes and which require insulin resistance to shut down whatever further caloric ingress it can practically do. Free fatty acids, a reasonable surrogate for the action of unmeasured insulin, are low so this suggests adipocyte sensitivity to insulin is high, hence the weight gain.
Weight gain in the alpha linolenic acid group was over 17 times that of the saturated fat group and 10 times that of the low fat group. Notice saturated fat protected (admittedly ns) against the weight gain seen on the low fat diet. The logic is obvious. What do the authors say? Well, I can find no mention in the discussion of this massive weight gain in the omega 3 group. Zilch. This is the quote from the only mention it gets, in the results section:
"Body weight at the end of the study was somewhat higher in db/db mice fed HF/3 compared with HF/S (Table 1)".
My emphasis.
There is no other mention of the hard fact that omega 3 fats are obesogenic. Also note that in relatively normal, non hyperglycaemic db/+ mice, the omega 3s are not obesogenic. Much the same as for non-fructose fed mice in the previous study.
Now look at the * I put in above. The omega 6 diabetic group LOST 2.4g. Ouch, at the core mitochondrial function level! How can this be? This needs no mention at all in the paper because p is greater than 0.05 (in the twisted stats used by the authors). But brownie points if you have noted the oddity about this particular group of mice.
Well done! Yes, in a group of 5 animals the standard deviation at the end of omega 6 feeding is 8.6. No other group had a standard deviation greater than 3 at any time. How do you get a standard deviation of 8.6? These are diabetic mice. Four gained weight, one became ill and this one lost a lot of weight. That's my guess, just trying to reverse engineer information out of the data supplied by a group of dissonant thinkers...
So, I went to an on-line standard deviation calculator and fed in various options where 4 mice gained some weight and one mouse lost a tonne of weight. Using a 2g gain for 4 possibly healthy mice and a 20g loss for the fifth poorly mouse we get four mice at 44g and one at 22g. This gives a mean weight at the end of the study of 39.5g to with an SD of just over 9. I think something like this is what happened. Would this group notice one skinny mouse in with four fat ones? Hahahahaha!
Summary: When PUFA are being oxidised in the mitochondria of adipocytes, those adipocytes are unable to resist the signal from insulin to distend with fat. The more double bonds in the PUFA has, the greater the effect. Linseed oil should be used for making varnish.
Peter
Raphi sent me this link early in the New Year. It’s nice. It demonstrates, at some level of complexity, that omega 6 PUFA at 8% of calories are obesogenic in mice, even if they are fed otherwise fat free CIAB. It’s all about endocannabinoid ligands and receptor activation. Potentially useful when folks get round to starting class actions against the cardiological community and any other health advisors warning against saturated fat. If you limit fat to 30% of calories and saturated fat to 10% you still have 20% PUFA/MUFA in your diet. That’s easily obesogenic. Your cardiologist made you fat. Sue now.
But all of this endocannabinoid stuff is what I call high level signalling. At the core mitochondrial level we know that omega 6 PUFA fail to limit insulin activity under situations where a saturated fat would shut down insulin mediated calorie ingress. In an adipocyte this means that, during oxidation of omega 6 PUFA, insulin continues to signal and fatty acids (and glucose) fall in to the adipocytes, stay there, and you get really hungry. Modified chemicals derived from this system of omega six fatty acids are overlaid on top of the core mitochondrial signalling. A modified derivative of arachidonic acid becomes an endocannabinoid ligand and makes you hungry and fat. The system takes something basic and develops an overlay of enormous complexity, this is what I call higher level signalling.
I hate higher level signalling. Give me the core process anyday.
On this front people may realise I have issues with omega 3 PUFA fats. From the ETC perspective they are worse than omega 6 PUFA and should be more obesogenic. But, in general they’re not. In fact there is a massive industry showing us how good they are for us. But there are suggestions that the core process which makes omega 6 PUFA obesogenic really do apply to the omega 3s. Bear in mind that we are only talking about linoleic and alpha linolenic acids here. Longer fatty acids go to peroxisomes for oxidation and have little influence on core mitochondrial processes, though they do perform a great deal of high level signalling. Here we go:
Sucrose counteracts the anti-inflammatory effect of fish oil in adipose tissue and increases obesity development in mice.
Notice the obesogenic effect of fish oil only shows when sucrose is present in the diet. Replacing sucrose with protein eliminates the effect. Fructose is an unstoppable source of cellular energy intake which needs insulin resistance to limit insulin signalling facilitated ingress of glucose. As insulin continues to act, fat cells sequester calories. Fish oil combined with sucrose is the worst, corn oil is intermediate and, without sucrose, none of the fats are obesogenic.
This makes me happy. I can see the core process at work, never mind what EPA and DHA say to g-protein coupled receptors.
There is another paper which shows a similar effect and I like it rather a lot because the cognitive dissonance, which shines through every word of the text, is rather entertaining. How can you get a life-sustaining source of funding if your data show that omega 3 PUFA are grossly obesogenic? They improve insulin signalling exactly as the ETC effects would predict. The cost of improved insulin responsiveness in adipocytes is obesity. Here we go again:
Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids.
The values to look at begin with the weight gain. All we have to do is to subtract weight at the start of the study period from weight at the end (perhaps the authors don't do arithmetic?). Low fat group gained a gram, added saturated fat group gained 0.6 g, added omega 6 group lost* 2.4g and omega 3 group gained 10.4g.
Ten point four grams.
These are db/db mice which lack a functional leptin receptor. They are diabetic and I feel their chronic hyperglycaemia represents a similar drive to obesity as the fructose loading in the last study, ie an unregulated source of calories which drop in to adipocytes and which require insulin resistance to shut down whatever further caloric ingress it can practically do. Free fatty acids, a reasonable surrogate for the action of unmeasured insulin, are low so this suggests adipocyte sensitivity to insulin is high, hence the weight gain.
Weight gain in the alpha linolenic acid group was over 17 times that of the saturated fat group and 10 times that of the low fat group. Notice saturated fat protected (admittedly ns) against the weight gain seen on the low fat diet. The logic is obvious. What do the authors say? Well, I can find no mention in the discussion of this massive weight gain in the omega 3 group. Zilch. This is the quote from the only mention it gets, in the results section:
"Body weight at the end of the study was somewhat higher in db/db mice fed HF/3 compared with HF/S (Table 1)".
My emphasis.
There is no other mention of the hard fact that omega 3 fats are obesogenic. Also note that in relatively normal, non hyperglycaemic db/+ mice, the omega 3s are not obesogenic. Much the same as for non-fructose fed mice in the previous study.
Now look at the * I put in above. The omega 6 diabetic group LOST 2.4g. Ouch, at the core mitochondrial function level! How can this be? This needs no mention at all in the paper because p is greater than 0.05 (in the twisted stats used by the authors). But brownie points if you have noted the oddity about this particular group of mice.
Well done! Yes, in a group of 5 animals the standard deviation at the end of omega 6 feeding is 8.6. No other group had a standard deviation greater than 3 at any time. How do you get a standard deviation of 8.6? These are diabetic mice. Four gained weight, one became ill and this one lost a lot of weight. That's my guess, just trying to reverse engineer information out of the data supplied by a group of dissonant thinkers...
So, I went to an on-line standard deviation calculator and fed in various options where 4 mice gained some weight and one mouse lost a tonne of weight. Using a 2g gain for 4 possibly healthy mice and a 20g loss for the fifth poorly mouse we get four mice at 44g and one at 22g. This gives a mean weight at the end of the study of 39.5g to with an SD of just over 9. I think something like this is what happened. Would this group notice one skinny mouse in with four fat ones? Hahahahaha!
Summary: When PUFA are being oxidised in the mitochondria of adipocytes, those adipocytes are unable to resist the signal from insulin to distend with fat. The more double bonds in the PUFA has, the greater the effect. Linseed oil should be used for making varnish.
Peter
Friday, January 15, 2016
Paignton Zoo
So funny that both articles come from Paignton Zoo in Devon. Has anyone contacted the victims of Lynne Garton's Going Ape "Evo Diet"? To tell them to knock off the fruit and live on raw kale leaves? Good enough for monkeys....Luckily Garton's stupidity seems to have done no permanent damage to it's victims, beyond 12 days of flatulence in the "study"!
Going ape.
Monkeys banned from eating bananas at Devon zoo.
Thanks to Amber O'Hearn via Faceache for the second link.
Peter
Going ape.
Monkeys banned from eating bananas at Devon zoo.
Thanks to Amber O'Hearn via Faceache for the second link.
Peter
Sunday, January 10, 2016
Not really much about swimming underwater
*****MAJOR ERROR********
Down in the comments section Mateusz has very kindly found the error in my arithmetic for me. It makes the whole of this post completely incorrect and requires a great deal of working through posts based on this conclusion to correct my mistake and the implications this has for blood supply and oxygen consumption.
Fats require around about 5% more O2 per ATP cf glucose.
With apologies to everyone.
I know I said (first paragraph) that I would take this post down in embarrassment if I'd made an arithmetical error but, on balance, I think it should stay as a warning, to me as much as anyone else.
So I’m going to leave this post up unchanged, with this edit, as a warming to the immense power of confirmation bias. There’s a lot to do.
*****MAJOR ERROR********
Just before I hit post: I think the arithmetic and the logic here are sound on a ball-park basis but if anyone can point out any major flaws I stand to be corrected and will take the post down in embarrassment. But this is so simple in concept that I don't see why it's not standard fare... Here we go.
In the comments after a previous post it became pretty obvious that several LC eating folks noted a significant improvement in their ability to breath-hold while running their metabolism on fat rather than on glucose. Although this is rather counter intuitive based on the RQ (more oxygen is required per unit CO2 generated when you oxidise fat compared to glucose) what matters is the generation of ATP per unit oxygen or ATP per unit CO2 produced. I started with oxygen. Arithmetic goes like this:
Glucose oxidation is simple. Six carbons give 2ATP from glycolysis and a mix of NADH and FADH2 from the TCA:
6(CH2O) + 6O2 = 6CO2 + 6H2O
RQ: CO2/O2 = 6/6 = 1.0
2 ATP + 10NADH + 2FADH2
A theoretical six carbon section of a chain of a fully saturated fatty acid gives this:
6(CH2) + 9O2 = 6CO2 + 3H2O
RQ: CO2/O2 = 6/9 = 0.67
15NADH + 6FADH2
Three of the FADH2s are from acetyl CoA turning the TCA, the other three are from beta oxidation. For PUFA a theoretical alternating sequence of single and double bonds yields this:
6(CH1.5) + 8.25 O2 = 6CO2 + 4.5 H2O
RQ: CO2/O2 = 6/8.25 = 0.73
15NADH + 3FADH2
The first step of beta oxidation for PUFA yields no FADH2, so we just have the three from the TCA. Assuming the ETC works efficiently we pump these protons from our hydrogen supply:
NADH = 12H+
FADH2 = 8H+
And, very crudely, let’s assume at complex V, ATP synthase, we have 4H+ = 1 ATP (not true IRL!)
So we can calculate protons pumped, what this is worth in ATP and combine this with the O2 needed (from the chemical equations above) giving:
Glucose protons
10NADH = 120 2FADH2 = 16, total = 136 H+
ATP 34 + 2 = 36
ATP-gluc/O2 = 6.00
Saturated fat protons
15NADH = 180 6FADH2 = 48, total = 228 H+
ATP = 57
ATP-sat/O2 = 6.33
PUFA protons
15NADH = 180 3FADH2 = 24, total = 204 H+
ATP = 51
ATP-pufa/O2 = 6.12
Clearly fatty acids are better at generating ATP per unit O2 consumed. If a 70kg person, at rest, is consuming 200ml of oxygen per minute to produce a given amount of ATP while burning glucose they should be able to maintain that same amount of ATP on less oxygen.
But the difference seems pretty small. How small?
Through sins of education I tend to think of O2 consumption for an anaesthetised, mechanically ventilated patient. That person needs about 200ml/min of oxygen.
200ml O2 gives 6.00 x10bw ATP if running on glucose (where 10bw is a crude scalar to whole body ATP needs). On saturated fat:
200ml O2 gives 6.33 x 10bw ATP
Or, more realistically:
190ml of O2 gives 6.00 x 10bw ATP on fat, equivalent to 200ml O2 used on glucose. An oxygen sparing effect of 10ml/min is underwhelming on first consideration. It’s a 5% improvement. But this should be maintained at VO2 max. When oxygen delivery is the limiting factor in performance, running on fat gives you a 5% advantage.
This is simple arithmetic applied to the most basic of biochemistry processes.
Is butter a performance enhancing drug?
Yes, provided it displaces carbohydrate.
Should folks with ischaemic problems eat butter?
Yes, provided it displaces carbohydrate.
Does it taste good?
Yes, unqualified.
Of course, once you add in ketones, magic starts to happen to the energy yield of ATP hydrolysis. Ketones are not as arithmetically simple as fatty acids but we all know, from Veech and D'Agostino's work, that magical indeed they are.
Peter
Oh, I calculated CO2 per unit ATP produced too. On carbs ATP/CO2 = 6.00 as you would expect but on saturated fat the amount ATP produced per unit CO2 evolved is 9.5. CO2 build up makes you breathe, you make less per minute on fats. Breath holding is, arithmetically thinking, expected to be easier running on saturated fat. This is what we find.
*****EDIT*****
Hans pointed out in comments that the TCA provides a molecule of GTP which can convert to ATP from each acetyl-CoA. This gives two extra ATP's per glucose and three more ATP's per six carbons from saturated fat. I can't be *rsed to re do the math, but you get the picture.
*****END EDIT*****
Down in the comments section Mateusz has very kindly found the error in my arithmetic for me. It makes the whole of this post completely incorrect and requires a great deal of working through posts based on this conclusion to correct my mistake and the implications this has for blood supply and oxygen consumption.
Fats require around about 5% more O2 per ATP cf glucose.
With apologies to everyone.
I know I said (first paragraph) that I would take this post down in embarrassment if I'd made an arithmetical error but, on balance, I think it should stay as a warning, to me as much as anyone else.
So I’m going to leave this post up unchanged, with this edit, as a warming to the immense power of confirmation bias. There’s a lot to do.
*****MAJOR ERROR********
Just before I hit post: I think the arithmetic and the logic here are sound on a ball-park basis but if anyone can point out any major flaws I stand to be corrected and will take the post down in embarrassment. But this is so simple in concept that I don't see why it's not standard fare... Here we go.
In the comments after a previous post it became pretty obvious that several LC eating folks noted a significant improvement in their ability to breath-hold while running their metabolism on fat rather than on glucose. Although this is rather counter intuitive based on the RQ (more oxygen is required per unit CO2 generated when you oxidise fat compared to glucose) what matters is the generation of ATP per unit oxygen or ATP per unit CO2 produced. I started with oxygen. Arithmetic goes like this:
Glucose oxidation is simple. Six carbons give 2ATP from glycolysis and a mix of NADH and FADH2 from the TCA:
6(CH2O) + 6O2 = 6CO2 + 6H2O
RQ: CO2/O2 = 6/6 = 1.0
2 ATP + 10NADH + 2FADH2
A theoretical six carbon section of a chain of a fully saturated fatty acid gives this:
6(CH2) + 9O2 = 6CO2 + 3H2O
RQ: CO2/O2 = 6/9 = 0.67
15NADH + 6FADH2
Three of the FADH2s are from acetyl CoA turning the TCA, the other three are from beta oxidation. For PUFA a theoretical alternating sequence of single and double bonds yields this:
6(CH1.5) + 8.25 O2 = 6CO2 + 4.5 H2O
RQ: CO2/O2 = 6/8.25 = 0.73
15NADH + 3FADH2
The first step of beta oxidation for PUFA yields no FADH2, so we just have the three from the TCA. Assuming the ETC works efficiently we pump these protons from our hydrogen supply:
NADH = 12H+
FADH2 = 8H+
And, very crudely, let’s assume at complex V, ATP synthase, we have 4H+ = 1 ATP (not true IRL!)
So we can calculate protons pumped, what this is worth in ATP and combine this with the O2 needed (from the chemical equations above) giving:
Glucose protons
10NADH = 120 2FADH2 = 16, total = 136 H+
ATP 34 + 2 = 36
ATP-gluc/O2 = 6.00
Saturated fat protons
15NADH = 180 6FADH2 = 48, total = 228 H+
ATP = 57
ATP-sat/O2 = 6.33
PUFA protons
15NADH = 180 3FADH2 = 24, total = 204 H+
ATP = 51
ATP-pufa/O2 = 6.12
Clearly fatty acids are better at generating ATP per unit O2 consumed. If a 70kg person, at rest, is consuming 200ml of oxygen per minute to produce a given amount of ATP while burning glucose they should be able to maintain that same amount of ATP on less oxygen.
But the difference seems pretty small. How small?
Through sins of education I tend to think of O2 consumption for an anaesthetised, mechanically ventilated patient. That person needs about 200ml/min of oxygen.
200ml O2 gives 6.00 x10bw ATP if running on glucose (where 10bw is a crude scalar to whole body ATP needs). On saturated fat:
200ml O2 gives 6.33 x 10bw ATP
Or, more realistically:
190ml of O2 gives 6.00 x 10bw ATP on fat, equivalent to 200ml O2 used on glucose. An oxygen sparing effect of 10ml/min is underwhelming on first consideration. It’s a 5% improvement. But this should be maintained at VO2 max. When oxygen delivery is the limiting factor in performance, running on fat gives you a 5% advantage.
This is simple arithmetic applied to the most basic of biochemistry processes.
Is butter a performance enhancing drug?
Yes, provided it displaces carbohydrate.
Should folks with ischaemic problems eat butter?
Yes, provided it displaces carbohydrate.
Does it taste good?
Yes, unqualified.
Of course, once you add in ketones, magic starts to happen to the energy yield of ATP hydrolysis. Ketones are not as arithmetically simple as fatty acids but we all know, from Veech and D'Agostino's work, that magical indeed they are.
Peter
Oh, I calculated CO2 per unit ATP produced too. On carbs ATP/CO2 = 6.00 as you would expect but on saturated fat the amount ATP produced per unit CO2 evolved is 9.5. CO2 build up makes you breathe, you make less per minute on fats. Breath holding is, arithmetically thinking, expected to be easier running on saturated fat. This is what we find.
*****EDIT*****
Hans pointed out in comments that the TCA provides a molecule of GTP which can convert to ATP from each acetyl-CoA. This gives two extra ATP's per glucose and three more ATP's per six carbons from saturated fat. I can't be *rsed to re do the math, but you get the picture.
*****END EDIT*****
Monday, December 28, 2015
Protons (43) Metformin in muscle
I've been meaning to post on this paper for a long time. It's old but not ancient (2006). The authors are interesting. Collier CA is first author and does not appear to have published anything else, ever. My guess is that it was her PhD which produced the paper and she dropped out of science at this point. Anyone who has tried to get funding for their first post doc will understand. Second author is Bruce CR and he has no other publications on metformin. Smith AC has one other publication on metformin but she wasn't looking at anything interesting from the Protons point of view. Last two authors are group leaders and have virtually zero publications on metformin.
So the lab dabbled in metformin for one PhD and lost either interest or funding. The paper has that feel to it. It looks preliminary, it has a few rough edges, the authors didn't appear to have known what the results were going to be before they started. Back in 2006 no one was thinking about mtG3Pdh or had any real idea of how metformin worked.
They used high doses of metformin and supra maximal doses of insulin on freshly isolated muscle tissue from healthy rats fed standard CIAB. So there is a simple black and white effect, nothing subtle. They looked at glucose oxidation and palmitate oxidation in acutely isolated soleus or epitrochlearis muscle. Soleus is a mixed fuel, oxidative muscle, epitrochlearis is glycolytic. Soleus is the one metformin works on. Here's the effect on palmitate oxidation:
One the left, metformin does nothing to suppress palmitate oxidation. No surprise there. On the right, insulin suppresses fatty acid oxidation.
That was one of the best findings in the paper. Never mind the lipophilic concept of obesity. Even if you keep your fatty acids outside of your adipocytes, insulin will suppress fatty acid oxidation in your soleus type muscles (i.e. an awful lot of them).
Metformin stops this happening and restores fatty acid oxidation. It does this for all of the reasons in the Protons thread which I won't repeat yet again except to say that, under metformin, insulin signalling can only be facilitated by fatty acid oxidation derived FADH2, not via mtG3Pdh FADH2.
The same happens for glucose oxidation:
Metformin alone does nothing to glucose oxidation in the absence of insulin but it blocks the small increase induced by supramaximal insulin.
If you want to suppress fatty acid oxidation in your muscles, insulin does this very nicely and metformin restores it. This was the most useful finding in the paper.
For whatever reason, they walked away from it.
Peter
Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle.
So the lab dabbled in metformin for one PhD and lost either interest or funding. The paper has that feel to it. It looks preliminary, it has a few rough edges, the authors didn't appear to have known what the results were going to be before they started. Back in 2006 no one was thinking about mtG3Pdh or had any real idea of how metformin worked.
They used high doses of metformin and supra maximal doses of insulin on freshly isolated muscle tissue from healthy rats fed standard CIAB. So there is a simple black and white effect, nothing subtle. They looked at glucose oxidation and palmitate oxidation in acutely isolated soleus or epitrochlearis muscle. Soleus is a mixed fuel, oxidative muscle, epitrochlearis is glycolytic. Soleus is the one metformin works on. Here's the effect on palmitate oxidation:
One the left, metformin does nothing to suppress palmitate oxidation. No surprise there. On the right, insulin suppresses fatty acid oxidation.
That was one of the best findings in the paper. Never mind the lipophilic concept of obesity. Even if you keep your fatty acids outside of your adipocytes, insulin will suppress fatty acid oxidation in your soleus type muscles (i.e. an awful lot of them).
Metformin stops this happening and restores fatty acid oxidation. It does this for all of the reasons in the Protons thread which I won't repeat yet again except to say that, under metformin, insulin signalling can only be facilitated by fatty acid oxidation derived FADH2, not via mtG3Pdh FADH2.
The same happens for glucose oxidation:
Metformin alone does nothing to glucose oxidation in the absence of insulin but it blocks the small increase induced by supramaximal insulin.
If you want to suppress fatty acid oxidation in your muscles, insulin does this very nicely and metformin restores it. This was the most useful finding in the paper.
For whatever reason, they walked away from it.
Peter
Metformin counters the insulin-induced suppression of fatty acid oxidation and stimulation of triacylglycerol storage in rodent skeletal muscle.
Saturday, December 12, 2015
Acetoacetate and arterial oxygen tension
This is very exciting. Remi forwarded it to me. He understands.
Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats
It’s from D'Agostino’s ketone group. Unless you are in to hyperbaric medicine you can ignore the bulk of the paper. Instead look at Fig. 3:
We're interested in the grey line in graph A with the triangle data points. How does enforced ketosis with an exogenous acetoacetate/betahydroxybutyrate precursor (but not when using a pure beta hydroxybutyrate precursor) raise arterial pO2 from the normal of 100mmHg to the rather spectacular high of 130mmHg?
This is fascinating and of genuine physiological significance. Not the raised arterial pO2 per se, more what it says about AcAc and metabolism. But never the less, how do you get a sustained increase in arterial pO2 by gavaging a with substance which is an AcAc precursor anyway? This is from the discussion:
“An unexpected finding was that BD-AcAc2 [the acetoacetate precursor] caused a significant and sustained increase in blood pO2 levels of ∼30%. It’s conceivable that these changes in PO2 result from BD-AcAc2-induced alterations in the neural control of autonomic regulation, including cardiorespiratory function (38). Further studies are needed to determine the specific contribution of BD-AcAc2 on brain O2 consumption, ventilatory drive, systemic blood pressure, and brain blood flow preceding CNS-OT.”
The finding was unexpected. There is no obvious explanation. It needs further study.
I love this. I’ll put on my anaesthetist’s hat and speculate.
The rats are breathing room air and there is nothing to suggest there has been any change in minute volume of breathing following treatment with the AcAc precursor. I think the effect possibly comes down to a decrease in tissue oxygen consumption under this drug derived ketone.
Aside: pO2 here is the partial pressure of oxygen in the arterial blood. This is only linked to oxygen content via the the oxygen-haemoglobin dissociation curve which is highly non linear. A change in pO2 from 100mmHg to 130mmHg is on the flat section of the curve and adds almost no oxygen carriage/delivery via haemoglobin. But it tells us things. End aside.
If you have a manoeuvre which decreases tissue oxygen consumption but leaves all else unchanged you will raise the partial pressure of oxygen in the alveoli within the lungs closer to the inspired concentration. This is because less is being taken up in to the blood, so more is left in those alveoli. Arterial blood leaving the lungs (in equilibrium with the alveolar pO2) will, therefore, have a higher partial pressure of oxygen too.
Equally, if you have lower oxygen consumption then the partial pressure of oxygen in the venous blood will be raised compared to normal tissue extraction, all other factors being unchanged. Again, it's because less is extracted, more is left. So there will be a higher venous oxygen partial pressure. Now, lungs are not 100% efficient. Some venous blood gets through and lowers the oxygen partial pressure in arterial blood. Higher oxygen partial pressure in venous blood means less effect on arterial blood pO2 through this lung inefficiency.
These are gross simplifications. John Nunn's Applied Respiratory Physiology, chapter 10 p242 onwards, "The oxygen cascade" has a little more detail. OK, a hell of a lot more, caveats included. Especially Fig 10.7.
Is this enough to explain D'Agostino's results? I don’t know. But an idea of whether I am correct would be given by taking a venous blood sample and measuring the venous pO2. The measured effect on arterial pO2 is large so you could possibly see a raised venous pO2 on a simple jugular vein sample without needing to try and get a pulmonary artery sample from a rat. That would give a “back of an envelope” assessment in little more time than it takes time to stick the sample through their blood gas analyser.
Equally, just stick a rat in respiratory chamber, gavage it with the acetoacetate precursor and measure its decrease in O2 uptake.
This finding has huge implications for managing any condition where oxygen delivery is compromised. Not the carotid pO2 of 130mmHg per se, this will have put very little more O2 on to haemoglobin than a pO2 of 100mmHg as stated. It's that decreased need for oxygen by the tissues which it signifies. Acetoacetate appears to allow tissues to function with a significantly reduced need for oxygen; that I find exciting. OK, I'm a bit strange but, well, that's me!
Peter
Summary: People climbing Everest should be in ketosis. With acetoacetate predominating.
Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats
It’s from D'Agostino’s ketone group. Unless you are in to hyperbaric medicine you can ignore the bulk of the paper. Instead look at Fig. 3:
We're interested in the grey line in graph A with the triangle data points. How does enforced ketosis with an exogenous acetoacetate/betahydroxybutyrate precursor (but not when using a pure beta hydroxybutyrate precursor) raise arterial pO2 from the normal of 100mmHg to the rather spectacular high of 130mmHg?
This is fascinating and of genuine physiological significance. Not the raised arterial pO2 per se, more what it says about AcAc and metabolism. But never the less, how do you get a sustained increase in arterial pO2 by gavaging a with substance which is an AcAc precursor anyway? This is from the discussion:
“An unexpected finding was that BD-AcAc2 [the acetoacetate precursor] caused a significant and sustained increase in blood pO2 levels of ∼30%. It’s conceivable that these changes in PO2 result from BD-AcAc2-induced alterations in the neural control of autonomic regulation, including cardiorespiratory function (38). Further studies are needed to determine the specific contribution of BD-AcAc2 on brain O2 consumption, ventilatory drive, systemic blood pressure, and brain blood flow preceding CNS-OT.”
The finding was unexpected. There is no obvious explanation. It needs further study.
I love this. I’ll put on my anaesthetist’s hat and speculate.
The rats are breathing room air and there is nothing to suggest there has been any change in minute volume of breathing following treatment with the AcAc precursor. I think the effect possibly comes down to a decrease in tissue oxygen consumption under this drug derived ketone.
Aside: pO2 here is the partial pressure of oxygen in the arterial blood. This is only linked to oxygen content via the the oxygen-haemoglobin dissociation curve which is highly non linear. A change in pO2 from 100mmHg to 130mmHg is on the flat section of the curve and adds almost no oxygen carriage/delivery via haemoglobin. But it tells us things. End aside.
If you have a manoeuvre which decreases tissue oxygen consumption but leaves all else unchanged you will raise the partial pressure of oxygen in the alveoli within the lungs closer to the inspired concentration. This is because less is being taken up in to the blood, so more is left in those alveoli. Arterial blood leaving the lungs (in equilibrium with the alveolar pO2) will, therefore, have a higher partial pressure of oxygen too.
Equally, if you have lower oxygen consumption then the partial pressure of oxygen in the venous blood will be raised compared to normal tissue extraction, all other factors being unchanged. Again, it's because less is extracted, more is left. So there will be a higher venous oxygen partial pressure. Now, lungs are not 100% efficient. Some venous blood gets through and lowers the oxygen partial pressure in arterial blood. Higher oxygen partial pressure in venous blood means less effect on arterial blood pO2 through this lung inefficiency.
These are gross simplifications. John Nunn's Applied Respiratory Physiology, chapter 10 p242 onwards, "The oxygen cascade" has a little more detail. OK, a hell of a lot more, caveats included. Especially Fig 10.7.
Is this enough to explain D'Agostino's results? I don’t know. But an idea of whether I am correct would be given by taking a venous blood sample and measuring the venous pO2. The measured effect on arterial pO2 is large so you could possibly see a raised venous pO2 on a simple jugular vein sample without needing to try and get a pulmonary artery sample from a rat. That would give a “back of an envelope” assessment in little more time than it takes time to stick the sample through their blood gas analyser.
Equally, just stick a rat in respiratory chamber, gavage it with the acetoacetate precursor and measure its decrease in O2 uptake.
This finding has huge implications for managing any condition where oxygen delivery is compromised. Not the carotid pO2 of 130mmHg per se, this will have put very little more O2 on to haemoglobin than a pO2 of 100mmHg as stated. It's that decreased need for oxygen by the tissues which it signifies. Acetoacetate appears to allow tissues to function with a significantly reduced need for oxygen; that I find exciting. OK, I'm a bit strange but, well, that's me!
Peter
Summary: People climbing Everest should be in ketosis. With acetoacetate predominating.
Monday, December 07, 2015
Protons (42) Metformin as the next epilepsy drug?
Some things which are written in stone are not quite as they seem. In a chat to karl about metformin/lactate in the brain I started thinking about the control of glucose derived calories being delivered to neurons. There is a general understanding that the brain does not use insulin signalling to control glucose entry to neurons, just as it doesn’t oxidise fatty acids. However we know that astrocytes certainly oxidise fatty acids to ketones and feed those ketones to the neurons, so the old chestnut about the "brain" not oxidising fatty acids is rather limited in its application. Does the same apply to glycolysis and glucose ingress? What about glial cells and insulin signalling?
So I pulled out this paper dated to August this year:
Insulin and IGF1 signalling pathways in human astrocytes in vitro and in vivo; characterisation, subcellular localisation and modulation of the receptors.
It’s a beautiful example of massively clever people who never ask the correct question. I opened the full text and slogged through reams and reams of alphabet soup about insulin signalling in astrocytes. The group are probably planning on maintaining funding by linking modifications of this "alphabet soup" to the development of type 3 diabetes, Alzheimer’s Disease. Great plan.
Of course personally I’m looking for changes in glucose metabolism related to insulin signalling. There is a sh!t load of mtG3Pdh in the mitochondria extracted from homogenised brain tissue and clearly it's doing something there. And that something, as far as I’m concerned, is related to linking glucose ingress to insulin signalling. The initiation and curtailing of insulin signalling in relationship to glucose flux.
After some time spent in the mire of alphabet soup I eventually searched the paper using “glucose” to see if I was missing some deep insight amidst the said alphabet soup.
No. glucose is only mentioned twice. The in-text the mention is irrelevant (talking about hepatic-like cell insulin resistance under fructose). The second mention is in a reference. This is a gem. Back in 1984 we knew this:
Insulin binds to specific receptors and stimulates 2-deoxy-D-glucose uptake in cultured glial cells from rat brain.
I would expect high levels of mtG3Pdh to be associated with very tight regulation of the glucose metabolism mediated through insulin signalling. Not in neurons. Neurons should use lactate. Glycolysis, especially the side-spur to the glycerophosphate shuttle, should be a pathway of last resort for neurons.
Not so in astrocytes. They should really, really tightly control the flux of glucose through themselves as they are the guardians of the neurons. They should meter insulin signalling to control lactate generation for supply to neurons.
Trying to link insulin signalling to Alzheimer’s Disease, without looking at glucose metabolism, leaves you wallowing in an alphabet soup with no way of generating a plan other than to develop some drug or other to block a downstream effect of one of those signalling molecules.
Will modifying the alphabet soup, without providing normoglycaemia, help anything? Well, yes, it will help generate funding.
Prevent AD?
Hahahahahahahaha.
This whole train of thought began with an email from karl linking to this is the editorial:
Fermenting Seizures With Lactate Dehydrogenase
Which discusses a particular paper (no abstract and one author disappeared between NEJM and PubMed, wtf????):
Inhibition of Lactate Dehydrogenase to Treat Epilepsy.
I've not read the text but the editorial is pretty clear about what they did. Does blockade of lactate dehydrogenase reduce seizures? Yes. But my suspicion is only if the astrocytes/glial cells are being driven hard through glycolysis either in tissue culture (at the "normal" high glucose levels used) or in mice fed crapinabag.
Summary: Lactate dehydrogenase feeds lactate from glial cells to neurons. This is Good. Blocking LDH will control seizures if they are being triggered by over supply of hyperglycaemia derived lactate from astrocytes. Metformin might do the same through all of the Protons logical reasons, ie it delays/limits insulin signalling until fatty acid oxidation replaces the glycerophosphate shuttle. By which time there will be increased beta oxidation leading to glial cell ketone generation... So, metformin SHOULD limit seizures if it promotes glial cell beta oxidation to ketones and reduces excess lactate by limiting insulin signalling. That metformin lowers blood glucose would help too.
Well, whoodathunkit?
Peter
Some text-hidden links:
Role of carnitine palmitoyltransferase I in the control of ketogenesis in primary cultures of rat astrocytes.
Roles and regulation of ketogenesis in cultured astroglia and neurons under hypoxia and hypoglycemia.
Metformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in mice.
So I pulled out this paper dated to August this year:
Insulin and IGF1 signalling pathways in human astrocytes in vitro and in vivo; characterisation, subcellular localisation and modulation of the receptors.
It’s a beautiful example of massively clever people who never ask the correct question. I opened the full text and slogged through reams and reams of alphabet soup about insulin signalling in astrocytes. The group are probably planning on maintaining funding by linking modifications of this "alphabet soup" to the development of type 3 diabetes, Alzheimer’s Disease. Great plan.
Of course personally I’m looking for changes in glucose metabolism related to insulin signalling. There is a sh!t load of mtG3Pdh in the mitochondria extracted from homogenised brain tissue and clearly it's doing something there. And that something, as far as I’m concerned, is related to linking glucose ingress to insulin signalling. The initiation and curtailing of insulin signalling in relationship to glucose flux.
After some time spent in the mire of alphabet soup I eventually searched the paper using “glucose” to see if I was missing some deep insight amidst the said alphabet soup.
No. glucose is only mentioned twice. The in-text the mention is irrelevant (talking about hepatic-like cell insulin resistance under fructose). The second mention is in a reference. This is a gem. Back in 1984 we knew this:
Insulin binds to specific receptors and stimulates 2-deoxy-D-glucose uptake in cultured glial cells from rat brain.
I would expect high levels of mtG3Pdh to be associated with very tight regulation of the glucose metabolism mediated through insulin signalling. Not in neurons. Neurons should use lactate. Glycolysis, especially the side-spur to the glycerophosphate shuttle, should be a pathway of last resort for neurons.
Not so in astrocytes. They should really, really tightly control the flux of glucose through themselves as they are the guardians of the neurons. They should meter insulin signalling to control lactate generation for supply to neurons.
Trying to link insulin signalling to Alzheimer’s Disease, without looking at glucose metabolism, leaves you wallowing in an alphabet soup with no way of generating a plan other than to develop some drug or other to block a downstream effect of one of those signalling molecules.
Will modifying the alphabet soup, without providing normoglycaemia, help anything? Well, yes, it will help generate funding.
Prevent AD?
Hahahahahahahaha.
This whole train of thought began with an email from karl linking to this is the editorial:
Fermenting Seizures With Lactate Dehydrogenase
Which discusses a particular paper (no abstract and one author disappeared between NEJM and PubMed, wtf????):
Inhibition of Lactate Dehydrogenase to Treat Epilepsy.
I've not read the text but the editorial is pretty clear about what they did. Does blockade of lactate dehydrogenase reduce seizures? Yes. But my suspicion is only if the astrocytes/glial cells are being driven hard through glycolysis either in tissue culture (at the "normal" high glucose levels used) or in mice fed crapinabag.
Summary: Lactate dehydrogenase feeds lactate from glial cells to neurons. This is Good. Blocking LDH will control seizures if they are being triggered by over supply of hyperglycaemia derived lactate from astrocytes. Metformin might do the same through all of the Protons logical reasons, ie it delays/limits insulin signalling until fatty acid oxidation replaces the glycerophosphate shuttle. By which time there will be increased beta oxidation leading to glial cell ketone generation... So, metformin SHOULD limit seizures if it promotes glial cell beta oxidation to ketones and reduces excess lactate by limiting insulin signalling. That metformin lowers blood glucose would help too.
Well, whoodathunkit?
Peter
Some text-hidden links:
Role of carnitine palmitoyltransferase I in the control of ketogenesis in primary cultures of rat astrocytes.
Roles and regulation of ketogenesis in cultured astroglia and neurons under hypoxia and hypoglycemia.
Metformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in mice.
Friday, November 27, 2015
Protons (41) Metformin in the liver
Just a brief note on metformin. No need for detailed analysis as there's not much to argue with. I think they are correct, even if we have differing views of the function of the glycerophosphate shuttle.
Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase
The paper makes a pretty good case for the action of metformin, at pharmacologically appropriate concentrations, as being to inhibit mtG3Pdh. At higher concentrations it undoubtedly inhibits complex I but its action at the glycerophosphate shuttle makes a great deal more sense. In the last post I looked at the inhibition of this shuttle as an inhibitor of glucose signalling which could be rescued by adequate fatty acid oxidation in the peripheral tissues. This too would undoubtedly be a critical action and I'll come back to it later.
I'd just like to emphasise first that the suppression of hepatic glucose output is also controlled by the redox state of the cytoplasm. In a normal liver cell a side spur of glycolysis drives enough electrons in to the ETC at mtG3Pdh to activate insulin signalling and concurrently reduces NADH while increasing NAD+. The rising level of NADH under metformin (due to blocking this oxidation of NADH) makes the conversion of lactate to pyruvate energetically impossible and so lactate derived gluconeogenesis stops on a redox basis. The conversion of glycerol to glucose via glycerol-3-phosphate is impossible using mtG3Pdh because metformin specifically blocks this enzyme. Some gluconeogenesis is quite possible via pyruvate, via alanine and other amino acids and, if you supply it, via dihydroxyacetone. But the paper suggests that redox change and enzyme inhibition underly the drop in hepatic glucose output seen with metformin. Fresh liver cells with or without metformin trying to generate glucose from various substrates gives us this picture:
Other parts of the paper were good too and I particularly enjoyed the forced change in lactate:pyruvate ratio in the culture medium section which mimicked metformin's action, but I think that's enough on modern views of metformin acting on the liver. I accept it works through inhibiting mtG3Pdh and subsequent change in redox status. Next proper post will be some ancient history from 9 years ago looking at muscles and metformin, where the Protons ideas from the last post get some support.
Peter
Summary: Metformin suppresses hepatic glucose output through decreased gluconeogenesis by inhibiting mtG3Pdh. Lots of evidence.
Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase
The paper makes a pretty good case for the action of metformin, at pharmacologically appropriate concentrations, as being to inhibit mtG3Pdh. At higher concentrations it undoubtedly inhibits complex I but its action at the glycerophosphate shuttle makes a great deal more sense. In the last post I looked at the inhibition of this shuttle as an inhibitor of glucose signalling which could be rescued by adequate fatty acid oxidation in the peripheral tissues. This too would undoubtedly be a critical action and I'll come back to it later.
I'd just like to emphasise first that the suppression of hepatic glucose output is also controlled by the redox state of the cytoplasm. In a normal liver cell a side spur of glycolysis drives enough electrons in to the ETC at mtG3Pdh to activate insulin signalling and concurrently reduces NADH while increasing NAD+. The rising level of NADH under metformin (due to blocking this oxidation of NADH) makes the conversion of lactate to pyruvate energetically impossible and so lactate derived gluconeogenesis stops on a redox basis. The conversion of glycerol to glucose via glycerol-3-phosphate is impossible using mtG3Pdh because metformin specifically blocks this enzyme. Some gluconeogenesis is quite possible via pyruvate, via alanine and other amino acids and, if you supply it, via dihydroxyacetone. But the paper suggests that redox change and enzyme inhibition underly the drop in hepatic glucose output seen with metformin. Fresh liver cells with or without metformin trying to generate glucose from various substrates gives us this picture:
Other parts of the paper were good too and I particularly enjoyed the forced change in lactate:pyruvate ratio in the culture medium section which mimicked metformin's action, but I think that's enough on modern views of metformin acting on the liver. I accept it works through inhibiting mtG3Pdh and subsequent change in redox status. Next proper post will be some ancient history from 9 years ago looking at muscles and metformin, where the Protons ideas from the last post get some support.
Peter
Summary: Metformin suppresses hepatic glucose output through decreased gluconeogenesis by inhibiting mtG3Pdh. Lots of evidence.
Friday, November 20, 2015
Protons (40) Living without the glycerophosphate shuttle
If we look at a mouse with a deletion of the gene for cytoplasmicG3Pdh we have a reasonable model for elimination of the glycerophosphate shuttle at the level were glycolysis would normally be used to drive reverse electron flow through complex I, to facilitate insulin signalling. These mice are remarkably normal and can swim in deep water, possibly not too happily, for at least 20 minutes with weights on their tails, i.e. they can exercise, at least if necessary to save their lives. They have markedly reduced levels of pyruvate and mildly elevated levels of lactate in muscle tissue compared to control mice.
It's the bottom two lines which I looked at. The lactate to pyruvate ratio reflects the NADH to NAD+ ratio within the cytoplasm, as Krebs puts it:
In a cell [H+] is a constant and K is also a constant (by definition).
So clearly we have a lot more NADH available in the knockout mouse, so more lactate gets formed from pyruvate, which becomes a minor player in the cascade of glycolysis to oxidative phosphorylation allowing lactate to take over. The authors of the mouse paper suggest that the lactate is expelled from the cells and that the Cori cycle, in the liver, is active to deal with the it. I’d prefer to think of lactate as being shunted directly to the mitochondria for use in the TCA.
If you subscribe to the view that the glycerophosphate shuttle is needed to provide NAD+ for glycolysis to proceed you might expect a few problems with glucose processing. There is undoubtedly an accumulation of metabolites upstream of the glycerophosphate shuttle and a depletion of those downstream but glycolysis does proceed. But from my point of view, with no glycerophosphate shuttle, there is nothing to allow the body to facilitate insulin’s drive to self activate using glucose. How do these mice cope?
They cope very well.
If you feel that tying a weight to the tail of a mouse and dropping it a beaker of water is a bit too crude, there are more sophisticated methods of inducing exercise. Worse than making obese people do cardio at any gym where fat shaming rules. It's possible to run a mouse to utter exhaustion and monitor its respiratory quotient while it runs. So you can see whether it burns predominantly glucose or fat and in what balance. You can also measure exactly how long it can run for, before it collapses at the level of exhaustion where it can no longer avoid an electroshock or two or three. Here are the core findings from sending mice to an electro-gym (the Thumb Tack Hypothesis taken to serious levels):
HeA are the knockout mice, By are the control mice. Knockout mice run harder and for longer than control mice. The rest of the graphs use the same coding, solid line is the control mice, dotted line the knockouts.
Taken from the RQ we can ask whether the knockout mice can oxidise glucose. Yes:
Pretty much as well as the control mice.
Can they oxidise lipid? Yes, somewhat better than can the control mice:
I think it is also worth noting that under marked but non-exhausting exercise that glycogen in the muscle of knockout mice does not fall, it does so in control mice:
These modified mice, which cannot use glycolysis to trigger insulin signalling, have a tendency to have MORE glycogen in their muscles (although p is greater than 0.05) at rest and they deplete it less under sustained near-maximal exercise. I'd guess insulin does signal.
It's also worth noting that blood lactate under the same conditions does not rise in the knockout mice whereas it does in control mice (p less than 0.05, yay!). My assumption is that lactate is being metabolised in the muscles of knockout mice and shunted to the liver for the Cori Cycle in control mice:
So what might be going on in these knockout mice? The requirement for insulin signalling is a modest amount of reverse flow of electrons through complex I, ie the CoQ couple must be reduced. The usual, here absent, technique is the glycerophosphate shuttle. But we can reduce the CoQ couple in other ways. My favourite way is via the oxidation of the FADH2 generated by metabolism of saturated fatty acids. How much FADH2 is needed to replace the glycerophosphate shuttle?
From the graph of lipid oxidation above we can see that knockout mice under exercise are oxidising somewhere around 45mg/kg/min of fat. The control mice are oxidising just over 30mg/kg/min. From the Protons perspective the increased fat oxidation is a requirement for normal insulin signalling and this insulin signal cannot limit fatty acid oxidation until the rate is almost 50% higher in the knockout mice than in those where the glycerophosphate shuttle works. These mice oxidise fat because insulin signalling is not being triggered by glycolysis. It also means that lipid oxidation has to be higher before it can trigger insulin resistance and cellular energy influx limitation.
We don't (as far as I know) have a drug to inhibit cytG3Pdh.
We do have one to inhibit mtG3Pdh, the other half of the glycerophosphate shuttle.
It's called metformin. Does metformin do the same thing as having a cytG3Pdh knockout does? Under exercise? In terms of getting the King Of the Mountains jersey in the Tour de France perhaps?
Possibly so.
Peter
Summary: Metformin blocks glycolysis triggered insulin signalling and cells replace this with FADH2 triggered insulin signalling from fatty acid oxidation (at ETFdh). This results increased fatty acid oxidation and in improved high intensity exercise ability. Oh, and I guess weight loss etc...
Aside. I think I might start sticking the refs from a post in at the end. There are times I can't remember in which post a paper was used, searching my own blog/hard drive might be easier if the author or a keyword are actually present rather than there just being a highlighted text field!!! The blog is getting a bit unwieldy.
Glycerol 3-phosphate dehydrogenase 1 deficiency enhances exercise capacity due to increased lipid oxidation during strenuous exercise
Mouse lacking NAD+-linked glycerol phosphate dehydrogenase has normal pancreatic beta cell function but abnormal metabolite pattern in skeletal muscle
The Redox State of Free Nicotinamide-Adenine Dinucleotide in the Cytoplasm and Mitochondria of Rat Liver
Metformin improves performance in high-intensity exercise, but not anaerobic capacity in healthy male subjects.
It's the bottom two lines which I looked at. The lactate to pyruvate ratio reflects the NADH to NAD+ ratio within the cytoplasm, as Krebs puts it:
In a cell [H+] is a constant and K is also a constant (by definition).
So clearly we have a lot more NADH available in the knockout mouse, so more lactate gets formed from pyruvate, which becomes a minor player in the cascade of glycolysis to oxidative phosphorylation allowing lactate to take over. The authors of the mouse paper suggest that the lactate is expelled from the cells and that the Cori cycle, in the liver, is active to deal with the it. I’d prefer to think of lactate as being shunted directly to the mitochondria for use in the TCA.
If you subscribe to the view that the glycerophosphate shuttle is needed to provide NAD+ for glycolysis to proceed you might expect a few problems with glucose processing. There is undoubtedly an accumulation of metabolites upstream of the glycerophosphate shuttle and a depletion of those downstream but glycolysis does proceed. But from my point of view, with no glycerophosphate shuttle, there is nothing to allow the body to facilitate insulin’s drive to self activate using glucose. How do these mice cope?
They cope very well.
If you feel that tying a weight to the tail of a mouse and dropping it a beaker of water is a bit too crude, there are more sophisticated methods of inducing exercise. Worse than making obese people do cardio at any gym where fat shaming rules. It's possible to run a mouse to utter exhaustion and monitor its respiratory quotient while it runs. So you can see whether it burns predominantly glucose or fat and in what balance. You can also measure exactly how long it can run for, before it collapses at the level of exhaustion where it can no longer avoid an electroshock or two or three. Here are the core findings from sending mice to an electro-gym (the Thumb Tack Hypothesis taken to serious levels):
HeA are the knockout mice, By are the control mice. Knockout mice run harder and for longer than control mice. The rest of the graphs use the same coding, solid line is the control mice, dotted line the knockouts.
Taken from the RQ we can ask whether the knockout mice can oxidise glucose. Yes:
Pretty much as well as the control mice.
Can they oxidise lipid? Yes, somewhat better than can the control mice:
I think it is also worth noting that under marked but non-exhausting exercise that glycogen in the muscle of knockout mice does not fall, it does so in control mice:
These modified mice, which cannot use glycolysis to trigger insulin signalling, have a tendency to have MORE glycogen in their muscles (although p is greater than 0.05) at rest and they deplete it less under sustained near-maximal exercise. I'd guess insulin does signal.
It's also worth noting that blood lactate under the same conditions does not rise in the knockout mice whereas it does in control mice (p less than 0.05, yay!). My assumption is that lactate is being metabolised in the muscles of knockout mice and shunted to the liver for the Cori Cycle in control mice:
So what might be going on in these knockout mice? The requirement for insulin signalling is a modest amount of reverse flow of electrons through complex I, ie the CoQ couple must be reduced. The usual, here absent, technique is the glycerophosphate shuttle. But we can reduce the CoQ couple in other ways. My favourite way is via the oxidation of the FADH2 generated by metabolism of saturated fatty acids. How much FADH2 is needed to replace the glycerophosphate shuttle?
From the graph of lipid oxidation above we can see that knockout mice under exercise are oxidising somewhere around 45mg/kg/min of fat. The control mice are oxidising just over 30mg/kg/min. From the Protons perspective the increased fat oxidation is a requirement for normal insulin signalling and this insulin signal cannot limit fatty acid oxidation until the rate is almost 50% higher in the knockout mice than in those where the glycerophosphate shuttle works. These mice oxidise fat because insulin signalling is not being triggered by glycolysis. It also means that lipid oxidation has to be higher before it can trigger insulin resistance and cellular energy influx limitation.
We don't (as far as I know) have a drug to inhibit cytG3Pdh.
We do have one to inhibit mtG3Pdh, the other half of the glycerophosphate shuttle.
It's called metformin. Does metformin do the same thing as having a cytG3Pdh knockout does? Under exercise? In terms of getting the King Of the Mountains jersey in the Tour de France perhaps?
Possibly so.
Peter
Summary: Metformin blocks glycolysis triggered insulin signalling and cells replace this with FADH2 triggered insulin signalling from fatty acid oxidation (at ETFdh). This results increased fatty acid oxidation and in improved high intensity exercise ability. Oh, and I guess weight loss etc...
Aside. I think I might start sticking the refs from a post in at the end. There are times I can't remember in which post a paper was used, searching my own blog/hard drive might be easier if the author or a keyword are actually present rather than there just being a highlighted text field!!! The blog is getting a bit unwieldy.
Glycerol 3-phosphate dehydrogenase 1 deficiency enhances exercise capacity due to increased lipid oxidation during strenuous exercise
Mouse lacking NAD+-linked glycerol phosphate dehydrogenase has normal pancreatic beta cell function but abnormal metabolite pattern in skeletal muscle
The Redox State of Free Nicotinamide-Adenine Dinucleotide in the Cytoplasm and Mitochondria of Rat Liver
Metformin improves performance in high-intensity exercise, but not anaerobic capacity in healthy male subjects.
Subscribe to:
Posts (Atom)